Arthroscopic Shavers & Cutters: A Surface-Engineering Guide for OEMs & Contract Manufacturers
Arthroscopic shavers and cutters face an unusual mix of demands: sharp, low-friction cutting, reliable suction in a tube-in-tube geometry, and—when reusable—hundreds of reprocessing cycles without staining, pitting, or particulate generation. This guide maps real-world failure modes (dulling, drag, debris, corrosion) to practical surface solutions and compares biocompatible chromium with common PVD options like DLC and TiN—within ISO 10993 and FDA reprocessing expectations.
How shavers/cutters work (and why edges fail)
Most arthroscopic shavers use a tube-within-a-tube design: a rotating inner cannula with a cutting window spins inside a fixed outer sheath that has a matching window. Suction pulls tissue into the window; the shearing action does the resection while fluid flow clears chips. Geometry at the distal tip (full-radius resector, oval, hooded, etc.) determines cutting behavior and debris evacuation.
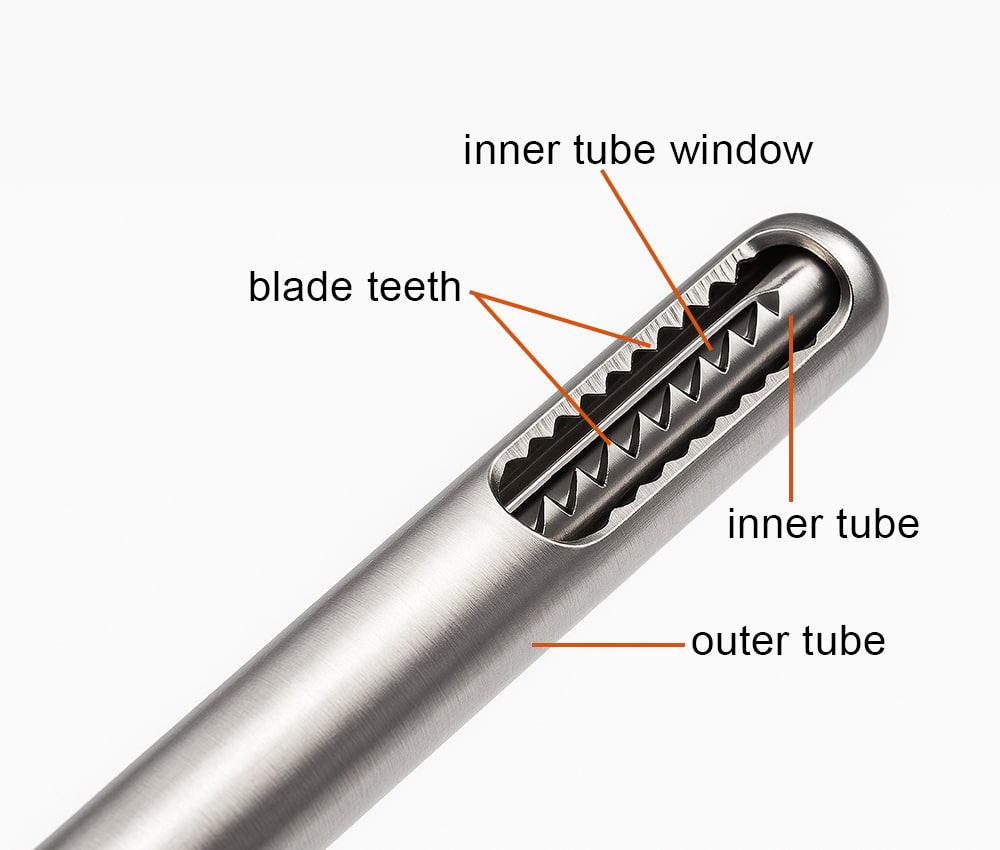
Substrates for shaver/cutter assemblies are most often martensitic (e.g., 420, 440A/440C) or precipitation-hardened stainless steels (e.g., 17-4 PH) for the cutting head—selected for heat-treat response, edge strength, and machinability. That said, mixed-alloy builds are common: for example, a 420 cutter head paired with an austenitic 304 (or 316) tube/sheath to balance sharpness with formability and baseline corrosion resistance. Surface preparation (electropolish, passivation) and the final finish/coating then govern friction, cleanability, and corrosion behavior under reprocessing; with tube-in-tube designs, thin, uniform deposits are critical to preserve clearances and suction performance.
Single-use vs reusable: different surface problems
Single-use blades
- Priorities: low friction during one case, edge integrity under brief torsion/impacts, minimal particulate.
- Typical strategy: optimized base finish + thin low-friction topcoat (often PVD) with cost control.
- View an example of a single-use shaver blade
Reusable blades
- Priorities: resistance to steam autoclave and detergents, stain/pit avoidance, long-term cleanability.
- Typical strategy: robust, cleanable metallic coating (e.g., biocompatible chromium), plus validated IFU compatibility.
Common failure modes and root causes
-
- Dulling & microchipping: edge micro-geometry damage from torsion, impact with bone, or suboptimal surface prep; high friction accelerates wear.
- Corrosion & staining post-autoclave: chloride-rich water, poor rinse quality, or aggressive chemistries; roughness peaks trap residues.

The autoclave machine pictured above can sterilize the most resistant pathogens, a result that can cause staining on materials not suitable for its harsh sterilization process.
- Galling between nested components: metal-on-metal contact in the tube-in-tube interface; insufficient lubricity and poor finish control.
- Debris/particulate generation: unstable films on sharp edges; inadequate adhesion; burr formation.
- Suction drag & clogging: boundary layer effects when surface roughness and wettability are mis-matched to the fluid path.
Coating options
Selecting a finish for shavers/cutters is about use case fit: substrate, reuse vs single-use, sterilization method, target edge life, and budget. The matrix below summarizes common approaches.
| Finish / Coating | Typical use | Sterilization compatibility* | Edge retention | Lubricity (tube-in-tube) | Dimensional impact | Notes |
|---|---|---|---|---|---|---|
| Electropolish + Passivation | Baseline for all stainless; often combined with topcoats | Steam, EO, H2O2 plasma | Moderate (substrate-limited) | Good if Ra/Rz tightly controlled | None | Foundation step; may not address galling alone |
| Biocompatible metallic chromium (ME-92®) | Reusable instruments needing cleanability & corrosion resistance | Steam, EtO, H2O2 plasma, radiation (e.g., gamma or electron beam), dry heat | High (stable metallic film) | High (low stick) | Low (thin, uniform deposit) | |
| DLC (a-C:H) | Single-use blades prioritizing sharpness & low friction | Steam (verify), EO, H2O2 plasma | High | High | Very low | Edge adhesion depends on prep; verify particulate |
| TiN / TiCN (PVD) | General cutting tools; color ID; moderate budget | Steam (verify), EO, H2O2 plasma | High | Medium–High | Very low | May “polish” with wear; verify adhesion on edge features |
*Always validate against your device’s IFU and reprocessing methods.
Sterilization & reprocessing compatibility
Reusable instruments must align with FDA’s guidance on validating reprocessing instructions and with current sterilization best practices (steam, EO, low-temperature H2O2 plasma). Surface finish affects detergent action, rinse quality, and residue removal. Design IFUs with the surface in mind—not just the geometry.
- Validate cleaning and sterilization cycles representative of real hospital practice.
- Control water quality (chlorides) and ensure thorough rinsing to reduce staining or pitting risk.
- If your supply chain uses EO sterilization, be aware of evolving regulatory attention on EO emissions and plan contingencies.
What ME-92® brings to shavers/cutters
ME-92® is a proven biocompatible chromium surface for surgical instruments and medical devices. For shavers/cutters, teams typically seek: cleanability after repeated reprocessing, stable corrosion resistance, and low tissue adhesion / low friction on the cutting window and tube-in-tube interface—while maintaining tight dimensions.
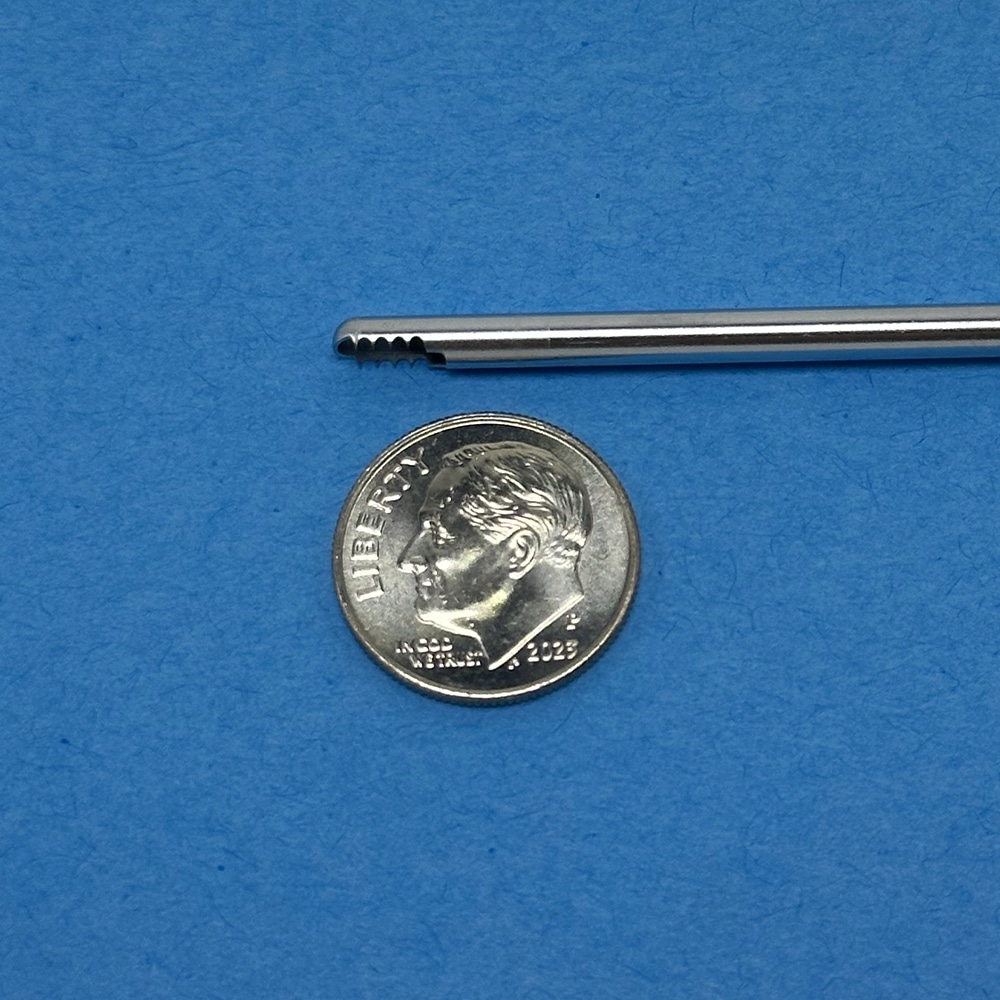
Arthroscopic shaver outer tube with cutting window and teeth next to a U.S. dime (17.91 mm diameter), illustrating how microns of coating thickness affect clearance.
Why thin, uniform deposits matter: the tube-in-tube clearances are small enough that microns of added thickness change fit and torque. ME-92® is specified as a thin, uniform metallic film to preserve clearances and suction performance.
- Thin, uniform metallic deposit (micron-scale) helps maintain tube-in-tube clearance.
- Cleanable surface with corrosion resistance under validated reprocessing cycles.
- Low-stick/low-friction behavior at the cutting window and sliding interface.
- Compatible with common sterilization modalities when validated on the device IFU.
For more on ME-92® biocompatible chromium, see our overview page: ME-92 Biocompatible Chromium.
Frequently Asked Questions
What’s best for single-use vs reusable blades?
Single-use often prioritizes low-friction films (e.g., DLC, TDC) and cost; reusable often prioritizes cleanability and long-term corrosion resistance (e.g., biocompatible metallic chromium). Validate for your device design and IFU.
How does surface finish affect suction performance?
Roughness and wettability at the inner tube and window influence boundary layer behavior and chip evacuation. Aim for consistent low-roughness and low-stick finishes where flow is critical; avoid steps and burr traps at the window edges.
Request a Shaver/Cutter Surface Spec Consult →
Free DFM review · 48-hour feasibility
References
- An overview of arthroscopic shaver mechanisms and geometry (tube-in-tube design).
- Single-use arthroscopic shaver blades and burrs are commercially available.
- ME-92® biocompatible chromium overview.
- FDA guidance for validating reprocessing of reusable devices (steam/cleaning/IFU).
- CDC disinfection and sterilization guideline (best practices and updates).
- Materials standards for surgical instruments (ASTM F899; ISO 7153-1:2016).
- Materials notes on common stainless grades used in surgical instruments (440 series; 17-4 PH).