
What is Pitting Corrosion?
A once sturdy metal surface gradually transforms into a landscape of tiny craters, as if it were the surface of a distant planet—this is the work of pitting corrosion. This type of corrosion, which forms small pits on metal surface, can concentrate in specific areas or distribute uniformly. Its partner in crime, crevice corrosion, occurs in areas of a part or assembly where moisture and contaminants are trapped, such as bolted assemblies or press-fit joints. These trapped environments lead to localized corrosion, often resulting in rust or corrosion between mating surfaces.
In this post—the latest in our series on metal failure mitigation—we’ll provide an overview of pitting and crevice corrosion, where they are most likely to occur, and how to prevent pitting corrosion and crevice corrosion using barrier coatings such as Armoloy TDC (Thin Dense Chromium).
Lurking Beneath: Pitting Corrosion, an Accelerating Threat
Pitting corrosion is commonly observed in alloys that typically form stable, passive oxide films, such as aluminum, stainless steel, and titanium. In severe cases, these pits can penetrate through the thickness of the metal. Crevice corrosion can accelerate due to the autocatalytic nature of the corrosion process, similar to pitting corrosion. For both mechanisms, once corrosion is initiated, it proceeds faster and faster due to the geometry of the crevice or pit.
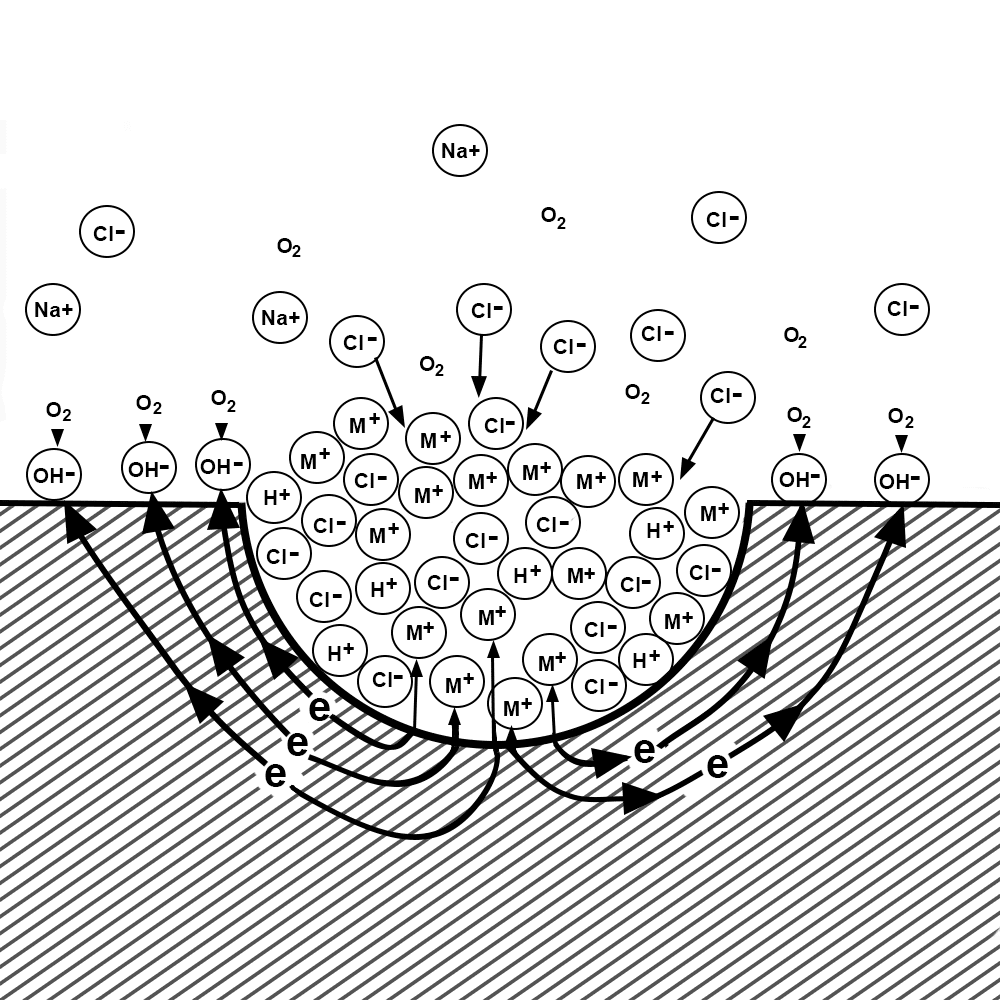
The shape of the pit itself causes corrosive species to concentrate inside the pit, accelerating the corrosion process.
Where Does Pitting and Crevice Corrosion Occur?
Pitting and crevice corrosion tend to occur in environments with a combination of moisture, oxygen, and corrosive agents like saltwater, chlorides, acidic solutions, or other aggressive chemicals. Furthermore, localized corrosion cells develop when the metal surface is not uniformly exposed to these factors. Some common scenarios where pitting and crevice corrosion occur include:
- Marine Environments. Pitting and crevice corrosion are particularly prevalent in marine environments due to the high salt concentration in seawater. Ships, offshore platforms, marine structures, and even coastal infrastructure like bridges, piers, railings, and buildings are susceptible to corrosion.
- Industrial Settings. Industries that utilize corrosive chemicals or acidic solutions are prone to pitting and crevice corrosion. These include chemical processing plants, petrochemical refineries, and wastewater treatment facilities, where metals are exposed to harsh chemicals and environmental conditions.
- Underground Pipelines. Buried metal pipelines, especially those carrying fluids containing chloride ions or other corrosive substances, are susceptible to pitting and crevice corrosion. The confined spaces around fittings, welds, or where the pipeline passes through soil with varying oxygen levels can promote these types of corrosion.
- Heat Exchangers and Condensers. Pitting and crevice corrosion can occur in heat exchangers and condensers where metal surfaces are in contact with cooling water or other fluids containing corrosive substances. The localized corrosion can be exacerbated by stagnant areas or where deposits accumulate.
- Medical Devices. Specific medical devices made of metal, such as implants or surgical instruments, may experience pitting or crevice corrosion when exposed to bodily fluids containing chloride ions or other corrosive substances.
Testing and Detection Methods for Pitting Corrosion
Testing methods for pitting corrosion, as outlined in ASTM G48, involve exposing samples to ferric chloride solution under controlled conditions to evaluate their resistance to corrosion. This standard provides six test methods that subject samples to varying temperatures and durations to promote either pitting or crevice corrosion. However, it’s essential to note that these tests can only determine real-world component life with extensive correlation testing. Their primary use is for ranking materials on the basis of their corrosion resistance.
One standard metric for ranking materials’ resistance to pitting corrosion is the Pitting Resistance Equivalent Number (PREN). This number considers the composition of alloys, precisely the amounts of chromium, molybdenum, tungsten, and nitrogen present. Alloys with higher PREN values, such as 316 stainless steel, are generally more resistant to pitting corrosion than those with lower PREN values. Real-world performance may vary, however, based on specific environmental conditions and other factors.
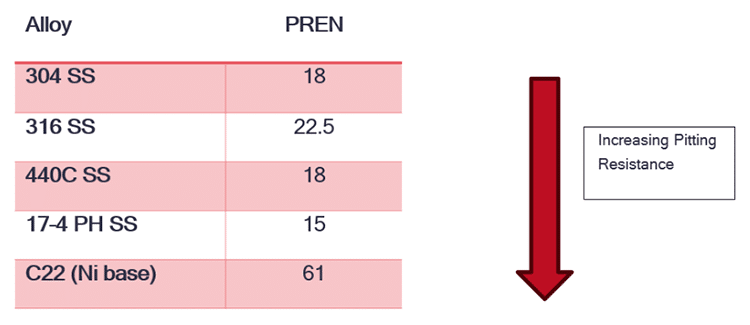
Several common alloys and their PREN’s
How to Prevent Pitting Corrosion in Stainless Steel and Metals
Mitigation strategies for pitting corrosion involve several approaches, including alloy selection, design practices, and the use of barrier coatings:
Importance of Alloy Selection
Choosing the right alloy is crucial in minimizing pitting corrosion. Alloys with higher PREN, such as stainless steel grades like 316 SS, offer better resistance to pitting corrosion. Aluminum alloys that are single-phase and relatively low in alloy content are also a preference. Higher PREN values indicate a higher resistance to pitting corrosion, making them suitable for applications where corrosion resistance is critical.
Role of Design Practices in Corrosion Prevention
Design optimization plays a significant role in preventing pitting corrosion. Minimizing crevices, reentrant angles, and areas that promote stagnation can help reduce the likelihood of pitting and crevice corrosion. By designing components and assemblies with smooth surfaces and proper drainage, the accumulation of moisture and contaminants reduce, thereby minimizing the risk of corrosion.
Use of Barrier Coatings for Protection
Barrier coatings such as paints, epoxies, and specialized coatings like Armoloy TDC can effectively protect against pitting corrosion. These coatings are a barrier between the metal surface and corrosive environments, preventing moisture and contaminants from reaching the metal substrate. Armoloy TDC, in particular, offers additional benefits such as wear resistance and low friction coefficients, making it suitable for applications requiring corrosion resistance and other properties.
Combining alloy selection, design optimization, and barrier coatings such as Armoloy TDC can significantly minimize the risks of pitting and crevice corrosion, ensuring the durability and reliability of metal components and structures in corrosive environments.
Engineered Solutions for Diverse Applications
Heighten performance, reduce maintenance costs, and enhance operational efficiency. Armoloy offers a diversified portfolio of plating technologies tailored to specific applications and environments. Take proactive measures and see how to prevent pitting corrosion today.