
In the intricate medical device and equipment manufacturing world, adherence to stringent quality systems such as ISO 10993 and FDA 21 CFR820 is paramount. Devices that come into direct or indirect contact with the human body are evaluated, explains the FDA, “for the potential for an unacceptable adverse biological response resulting from contact of the component materials of the device with the body.”
As a result, the choice of medical device materials and coatings plays a pivotal role in meeting regulatory requirements. Despite the strength of stainless steel and aluminum — materials used for everything from surgical implants to orthopedic tooling — corrosion and wear occur partly due to repeated cleaning, decontamination, and sterilization procedures. In this post, we’ll look at the industry challenges and standards for sterilization and how biocompatible coatings from ME-92 and Armoloy Southeast protect and extend product life.
Sterilization Challenges for Medical OEMs
Medical equipment manufacturers face a complex web of quality control and regulatory processes to ensure a repeatable process that meets or exceeds compliance standards. Contamination risk is critical across all design stages, from prototype and production to packaging and delivery. Leading medical OEM considerations include:
Material Compatibility
Common sterilization methods include steam sterilization, ethylene oxide (EtO) sterilization, radiation (e.g., gamma or electron beam), and dry heat. However, not all materials are compatible with every sterilization method. For instance, heat-sensitive materials cannot undergo steam sterilization. Manufacturers must ensure that the chosen sterilization process does not damage the device or alter its material properties.
Design Complexity
Modern medical devices often have complex designs with intricate parts or embedded electronics; ensuring complete sterilization while maintaining surface and performance integrity after repeated cycles is challenging.
Bacterial Adherence, Residue, and Toxicity
Unlike EtO and other chemical sterilization methods, steam autoclaving, for example, doesn’t leave toxic residues on the sterilized items — crucial for patient safety. However, metal instruments can corrode over time due to repeated exposure to steam.
Regulatory Compliance
Complying with ISO 10993 biocompatibility standards ensures routine control of sterilization methods to meet FDA and other regulatory requirements, a key component of all OEM processes. However, validating the efficacy of sterilization processes, especially for new or modified devices, is a complex task.
Scalability and Cost
Balancing the cost-effectiveness and scalability of sterilization processes with all the other factors — materials compatibility, design complexity, safety and efficacy — is one of the most complex challenges.
As with any process, there are pros and cons to various sterilization procedures, several of which we’ve outlined below. Addressing these challenges requires balancing sterilization and regulatory requirements with specific medical equipment design needs.
Sterilization Factors/Considerations for Medical Devices and Surgical Instruments
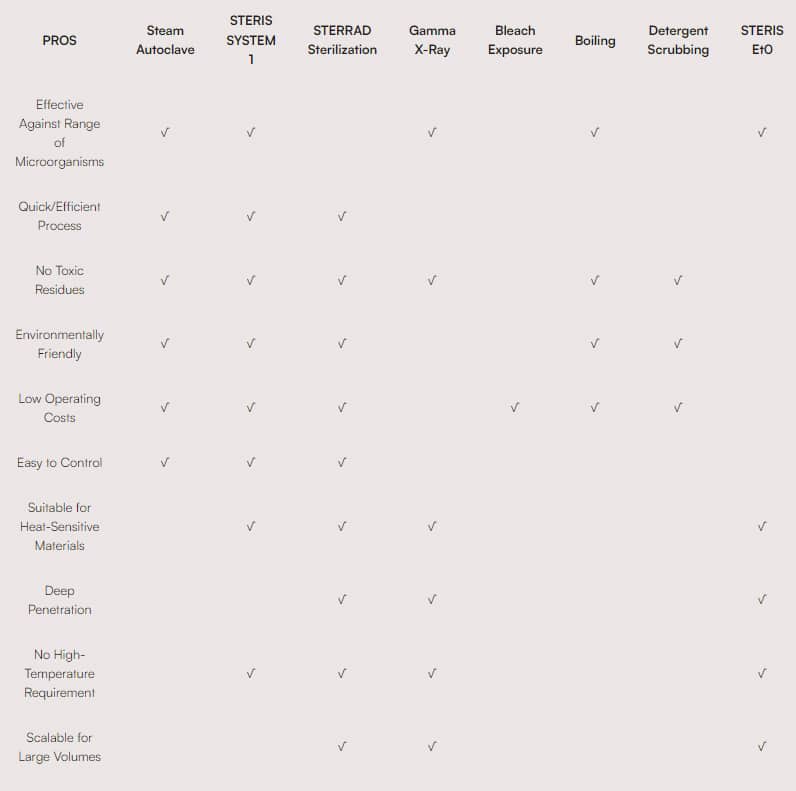
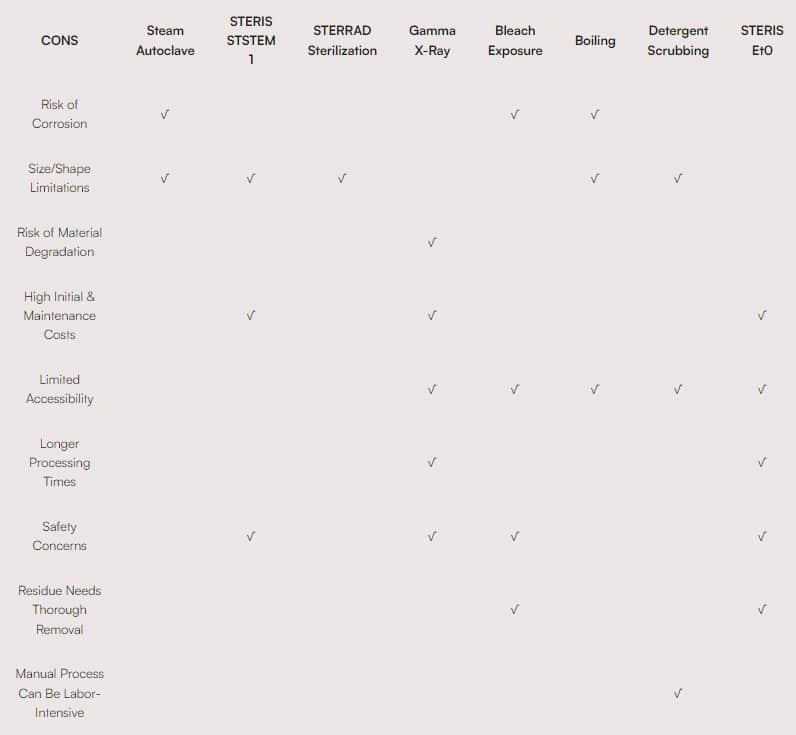
For more information, please visit individual equipment manufacturer’s website.
Biochrome Coatings: Durability, Safety, and Effectiveness
Biocompatible chromium coatings for stainless steel and aluminum medical devices, components, and surgical tools can significantly address some of the challenges and limitations of sterilization procedures. ME-92® and AL-COAT® for stainless steel and aluminum medical devices, components, and surgical instruments also address some of the challenges and constraints of sterilization procedures:
Combating Corrosion
ME-92® biochrome coatings provide an additional layer of protection against corrosion, which is especially important for methods involving moisture, such as steam autoclave and boiling, or corrosive chemicals, like bleach exposure. Arresting corrosion from sterilization and laser marking while maintaining a new appearance, ME-92® enhances the longevity and maintains the integrity of surgical instruments and medical devices.
Working with Complex Geometries and Intricate Designs
Different sterilization methods, from Steam Autoclave and STERRAD Sterilization to boiling and detergent scrubbing, run the risk of microorganisms hiding in crevices or uneven surfaces. Uniform ME-92® chromium coating can mitigate some of the sterilization challenges posed by complex device geometries, including those with intricate designs that are hard to sterilize completely. Furthermore, the coating is highly ductile and will not separate from the base material even when twisted, flexed, or impacted without intermediate layering.
Reducing Residue and Toxicity Concerns
ME-92® chromium coatings are resistant to chemical reactions and do not interact with EtO gas, thus reducing the risk of toxic residues adhering to the device surface after STERIS EtO sterilization.
Promoting Safety for Patient Contact
The first to perform and pass the Tripartite Guidelines Testing for Biocompatibility, ME-92 Operations biochrome coatings reduce bacterial adherence, facilitate easier cleaning and decontaminating, and maintain the surface integrity of medical equipment and surgical tools after repeated sterilization cycles. ME-92 has met or exceeded the requirements of the ISO 10993 family of industry standards for a biocompatible coating.
No Limits with ME-92
ME-92® precision manufacturing and finishing is a strategic product enhancement for medical device manufacturers. Engineered to meet each customer’s specific requirements, ME-92® addresses specific limitations of sterilization methods and adds value by improving performance, safety, and durability. Discover what we can do for you.