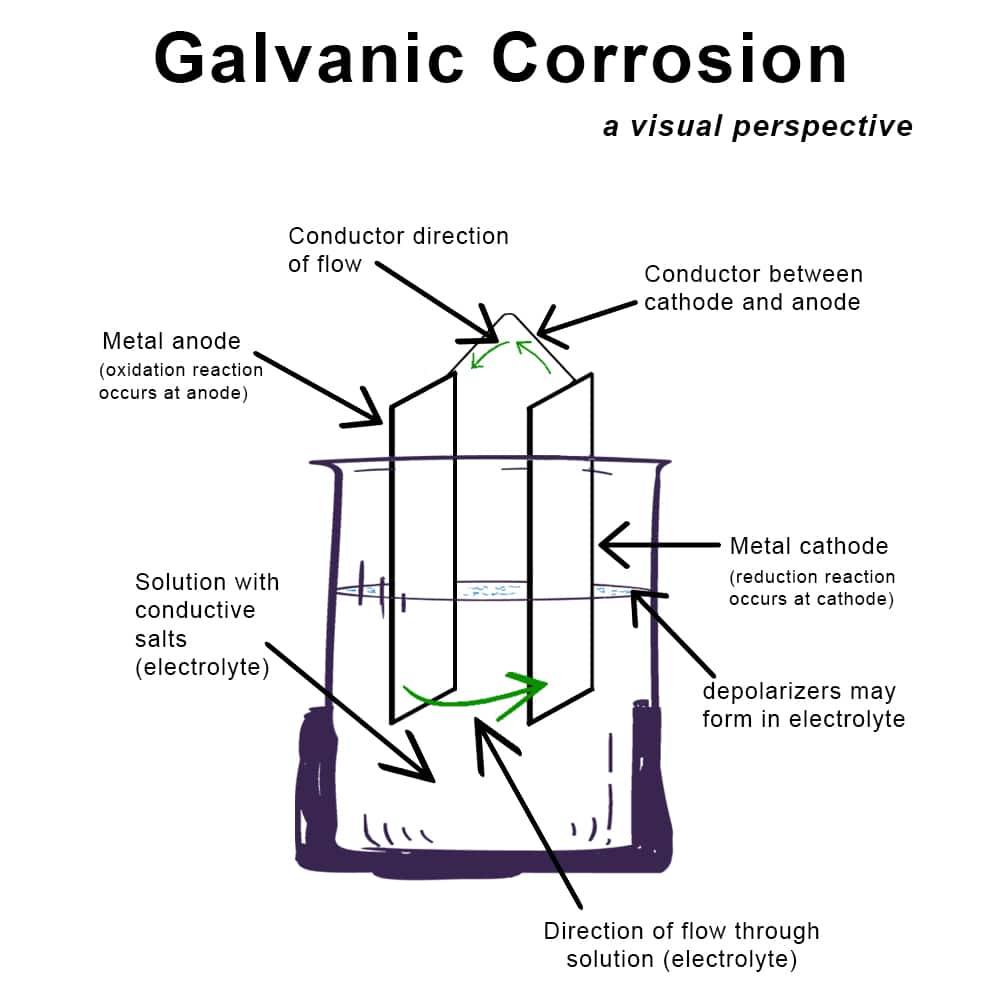
Galvanic corrosion occurs when two dissimilar metals come into electrical contact in the presence of an electrolyte (like moisture or saltwater). The more anodic metal corrodes while the more cathodic metal remains protected. Below are common metals and how they behave in galvanic pairs:
- Zinc: Highly anodic — corrodes rapidly when in contact with stainless steel, copper, or brass.
- Aluminum: Anodic — vulnerable when paired with more noble metals like copper or stainless steel.
- Carbon Steel / Low-Alloy Steel: Moderately active — can corrode when coupled with cathodic metals such as bronze or stainless steel.
- Copper: Cathodic — promotes corrosion in less noble metals like zinc, aluminum, or galvanized steel.
- Brass & Bronze: Cathodic — often causes corrosion in aluminum and steel when in direct contact.
- Stainless Steel: Highly cathodic — accelerates corrosion of adjacent anodic metals like zinc or aluminum.
Galvanic Series: This electrochemical ranking lists metals from most anodic (active) to most cathodic (noble). The further apart two metals are in the series, the higher the risk of galvanic corrosion when they touch.
Prevention Tips:
- Isolate dissimilar metals: Use gaskets, bushings, or non-conductive barriers to break electrical contact.
- Apply surface protection: Use protective coatings to shield one or both metals from electrolyte exposure.
- Select compatible materials: Choose metals that are close together on the galvanic series to minimize potential difference.
Knowing how different metals interact can help you prevent galvanic corrosion and extend the life of your equipment.