Tribocorrosion is the result of two simultaneous forces acting on a material’s surface: mechanical wear and chemical or electrochemical corrosion. Together, these forces accelerate degradation beyond what either would cause alone. The term combines tribology (the study of friction, wear, and lubrication) with corrosion, reflecting how these interactions can produce a synergistic effect—material breakdown that’s faster and more severe than the sum of its parts.
Though relatively new as a formal field of study, tribocorrosion has long impacted a wide range of systems—from industrial pumps and mining equipment to biomedical implants and nuclear reactor components. In many cases, tribocorrosion drastically shortens service life and raises safety concerns. At the same time, certain industries harness it intentionally—for example, in semiconductor polishing or reactive lubricant machining—where controlled wear-corrosion effects are used to advantage.
The following sections explore the science behind tribocorrosion, from its underlying mechanisms to practical examples and ongoing research efforts:
-
- What is Tribocorrosion?
- Key Mechanisms of Tribocorrosion
- Types of Tribocorrosion
- Tribocorrosion Testing Methods
- Examples from Real World Applications
- Current Research Themes
What is Tribocorrosion?
Tribocorrosion refers to material degradation caused by simultaneous wear and corrosion processes. A classic definition (ASTM G40-10b) describes it as a form of surface alteration due to the joint action of relative mechanical contact and a chemical reaction, where the total effect is different (often worse) than either process alone. In essence, whenever two surfaces in relative motion are exposed to a corrosive environment, tribocorrosion can occur.
- Wear in this context is a mechanical process: the removal or deformatin of material due to frictional contact (sliding, rolling, or impact).
- Corrosion is a chemical or electrochemical process: the reaction of mateiral (usually metal) with its environment (e.g. oxidation, dissolution in a fluid).
These processes can mutually accelerate each other. Wear can strip away protective oxide films or coatings, exposing fresh reactive metal to the environment and accelerating corrosion. Conversely, corrosion can weaken a material’s surface (making it softer or more brittle), which speeds up mechanical wear. This mutual enhancement is why tribocorrosion often causes more damage than the sum of “wear-only” plus “corrosion-only” damage – a clear sign of synergy. Researchers sometimes distinguish between corrosion-accelerated wear and wear-accelerated corrosion, but in practice both contribute simultaneously in tribocorrosion. A related term you’ll encounter is “mechanically assisted corrosion”, essentially synonymous with tribocorrosion.
Fretting corrosion is an early-recognized example: tiny oscillatory motions between metal surfaces (like in a bolted joint or implant) cause wear debris and disrupt surface films, while the environment induces corrosion – together leading to damage far worse than simple rubbing or rusting alone. Tribocorrosion’s effects are especially pronounced in metals that rely on passive oxide films for corrosion resistance (e.g. stainless steels, titanium alloys). Once the protective film is repeatedly worn off, the underlying metal can corrode rapidly until the film re-forms – and if the wear is continuous, the metal may remain in an actively corroding state. This is why even highly “corrosion-resistant” metals can suffer severe damage under tribocorrosive conditions.
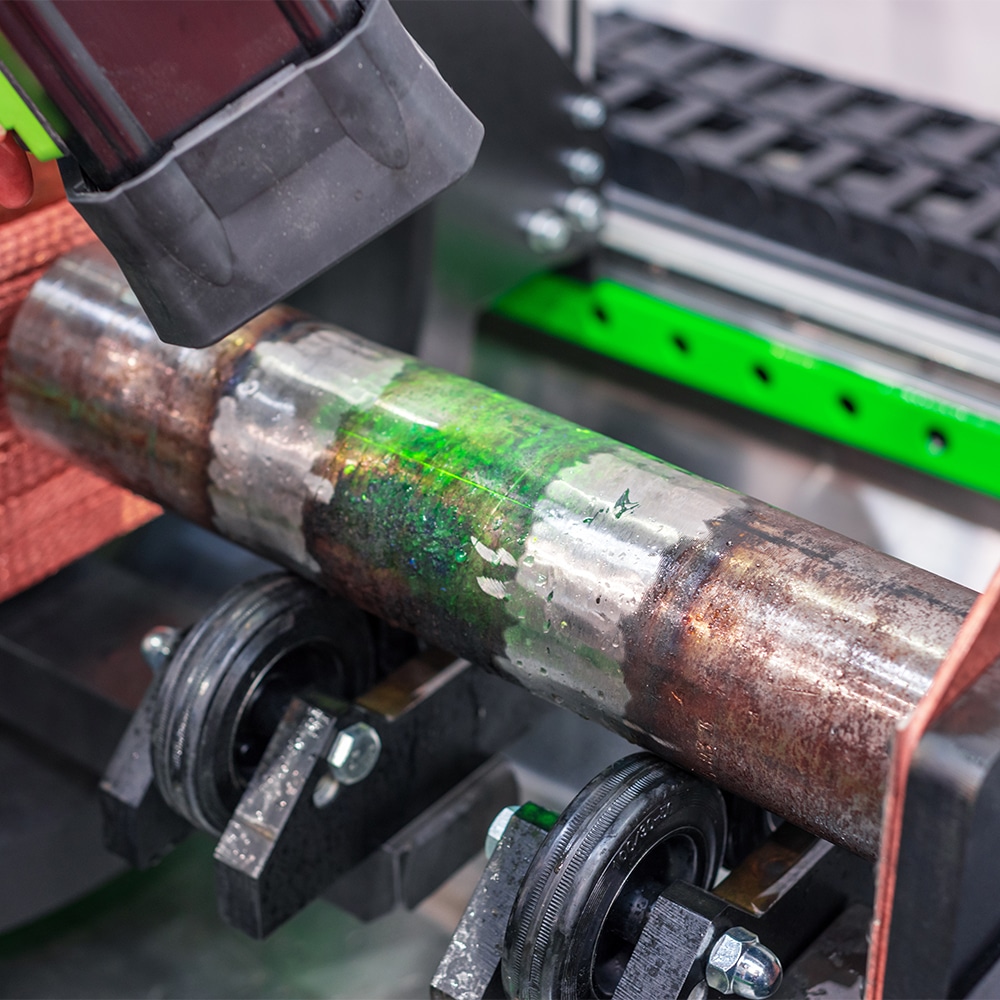
Fretting wear can be seen on this metal shaft as it’s being assessed for severity of damage.
In summary, tribocorrosion is the coupling of wear and corrosion in a way that degrades materials faster or in a new ways. It demands attention wherever equipment contacts a corrosive medium under motion or load – from the human body (implants) to oceans (ship parts) to industrial plants.
Key Mechanisms of Tribocorrosion
Tribocorrosion involves complex mechanisms that intertwine mechanical and electrochemical phenomena. Some of the key mechanisms and concepts include:
Passivation and Depassivation Cycles
Many metals (like stainless steel, Ti, Al alloys) form thin oxide layers that passivate their surface and slow down corrosion. Mechanical action (sliding, abrasion, impact) can rupture this passive film continuously. Each time the film breaks, fresh metal is exposed to corrosive attack until the film re-forms (repassivation). If the wear rate exceeds the repassivation rate, the metal remains effectively “active,” corroding much faster than normal. This repetitive film removal and reformation is central to tribocorrosion of passive metals. The net material loss comes from both mechanical wear (lost particles) and corrosion dissolution (metal ions going into solution). Often, the total loss is far greater than the sum of each process alone, due to the positive feedback loop between wear and corrosion. (In rare cases, slight wear might remove corrosive build-up, giving an antagonistic effect that reduces corrosion locally, but generally synergy dominates.)
Wear-Corrosion Synergy
The hallmark of tribocorrosion is synergy. Wear processes (like abrasion, adhesion, fretting, fatigue) and corrosion processes (like oxidation or acid attack) interact. For example, consider sliding wear in a corrosive fluid: frictional heating and micro-plastic deformation can make the surface more electrochemically active, while corrosion pits or hydrogen embrittlement from the fluid make the metal easier to wear. The result is a non-linear increase in damage. Engineers quantify this by separately measuring pure wear, pure corrosion, and combined tribocorrosion, then calculating a synergy factor (per standards like ASTM G119). A synergy factor >1 indicates the combined effect is greater than additive. This synergy is why tribocorrosion can be so destructive in practice.
Hydrogen embrittlement is seen on an uncoated steel bar, caused by the synergy between fatigue wear and oxidation
Material Transfer and Tribofilms
In some tribocorrosion scenarios, wear debris can oxidize or react with the environment to form new compounds on the surface (tribofilms). For instance, at high temperatures, oxide debris may sinter into protective glazed layers on the wear track. These oxide “glazes” can actually reduce further wear – an interesting case where a tribocorrosion process mitigates damage. In more common cases (e.g. metal-on-metal in saline), wear debris may form corrosive oxidized slurries or third-body particles that abrade the surface further. The composition and behavior of such tribofilms are an active research area, as they can either accelerate damage or, occasionally, provide some protection.
Electrochemical Response to Mechanical Stimuli
The moment a passive surface is scratched, the metal’s open-circuit potential can drop (indicating active dissolution). If one measures the corrosion current during rubbing, it often spikes during slip events due to film breakdown. Once motion stops, the current decays as the film heals. Tribocorrosion involves this dynamic electrochemical response to mechanical events. Parameters like the repassivation rate, the time to restore passivity, and the extent of anodic current during sliding all describe how aggressively a material corrodes under wear. Harder materials or those with faster repassivation (like titanium and its alloys) may endure tribocorrosion better than softer or slower-passivating ones. Conversely, applied potentials (e.g. galvanic or anodic bias) can influence how much corrosion occurs during wear. Controlling these factors is key to understanding tribocorrosion behavior.
In short, tribocorrosion mechanisms revolve around how mechanical actions (rubbing, impact, abrasion) continuously disturb a material’s surface chemistry, and how the environment’s chemistry in turn influences mechanical degradation. It’s a true multidisciplinary problem – involving frictional physics, materials science (surface films, microstructure), and electrochemistry all at once.
Types of Tribocorrosion
Tribocorrosion can manifest in several forms depending on the nature of the mechanical motion and environment. Common types (sometimes overlapping) include:
Sliding Wear-Corrosion (Corrosive Sliding)
This refers to classic scenarios of two surfaces sliding against each other in a corrosive medium. For example, a steel shaft rotating in saltwater or an acidic solution will experience regular frictional wear and corrosion. The combined effect can be termed corrosion wear or sliding tribocorrosion. Over time, grooves, pits, and material loss appear on the sliding surfaces due to the synergy of rubbing and rusting. Many machinery parts (gears, bearings, seals) in chemical or marine environments suffer this form of tribocorrosion.
Fretting Corrosion
Fretting is a special case of sliding with very small amplitude oscillatory motion, often at contact interfaces that are supposed to be static (like bolted joints, press-fits, or modular implant connections). The small repeated micromovements produce fine wear debris and disrupt protective films. Meanwhile, exposure to air or liquid causes oxidation of the freshly exposed material. The result is often a reddish-black oxide residue at the interface and surface pitting – classic fretting corrosion damage. Fretting tribocorrosion is common in orthopedic implants (where a stem and bone interface can microslip) and in aerospace or automotive parts that vibrate. It’s particularly insidious because the motions are so small they might go unnoticed until significant damage accumulates.
Severe fretting corrosion is noticeable on this bearing box, as evident of the reddish-black oxide residue seen.
Abrasion-Corrosion
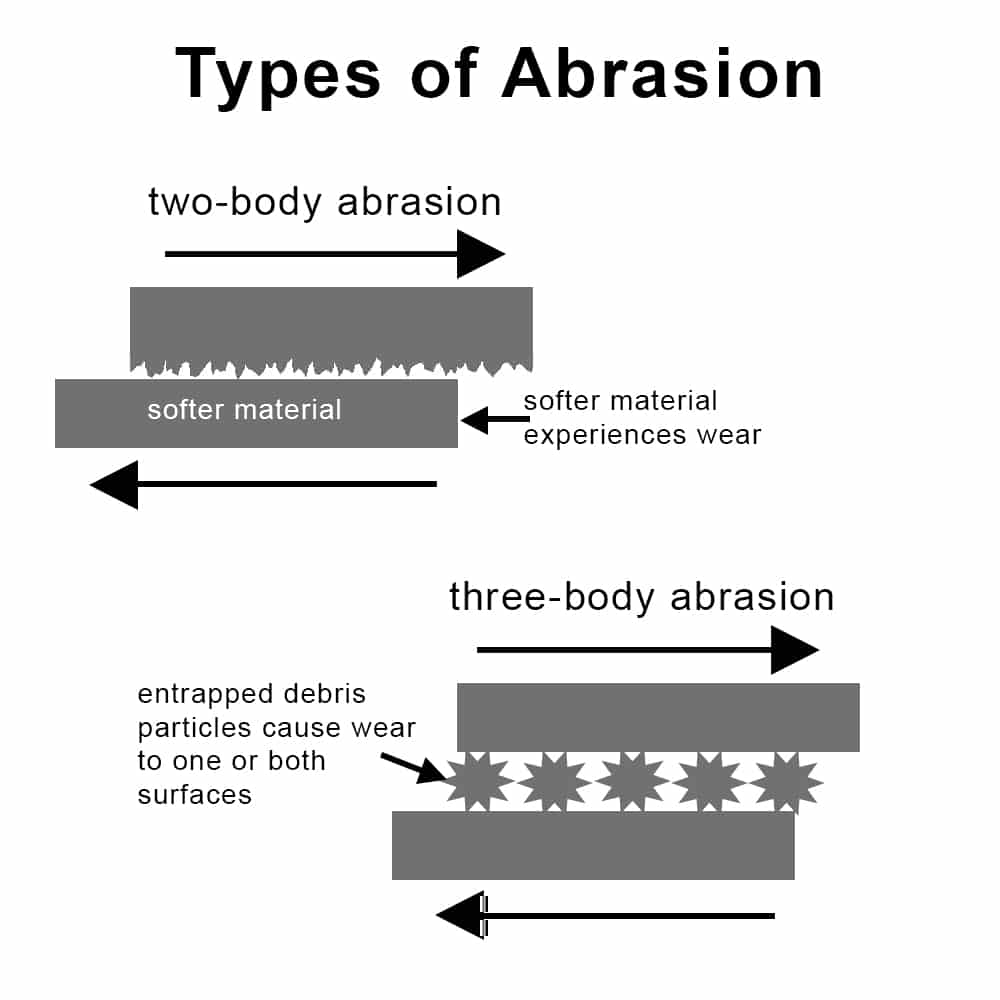
This occurs when hard particles or abrasives are present and cause three-body wear in a corrosive environment. Imagine sand or grit circulating in a fluid through a pump or pipe: the particles physically abrade surfaces (like sandpaper), and the fluid corrodes the freshly exposed metal. This abrasion-corrosion combination is often seen in mining slurry pumps, pipelines carrying ore or sand in water, and wear of coatings by hard debris. The abrasive action continually exposes new material, preventing any stable passive film from protecting the surface. Abrasion-corrosion is very damaging to protective coatings and paints as well, as scratches let corrosive agents penetrate.
The schematic above provides a comparison between two-body abrasion (wear without corrosion), and three-body abrasion (wear accelerated by corrosion debris).
Erosion-Corrosion
Erosion-corrosion involves rapid impingement or flow that mechanically wears a surface while also causing corrosion. Typical examples are: high-speed liquid flow eroding a pipe bend, slurry of particles impinging on a valve, or cavitation bubble collapse near a ship propeller.
Mechanically, material is removed by micro-cutting, fatigue, or bubble implosion pressure, and the concurrent exposure to a corrosive fluid accelerates rust or dissolution. Cavitation erosion-corrosion on ship propellers or pump impellers is a well-known problem – the implosion of vapor bubbles eats away metal and the seawater chemistry furthers the attack.
Erosion-corrosion can cause a characteristic pitting or scalloping of surfaces and often occurs in tandem with flow-accelerated corrosion (FAC) in power plant piping. (FAC is sometimes classed separately, focusing on the chemistry of fast-flow water removing protective oxide, but it’s related.)
Heat exchanger systems are no stranger to erosion corrosion, as the one pictured above experienced a long service life of constant, high speed media flow.
Contact Fatigue Corrosion
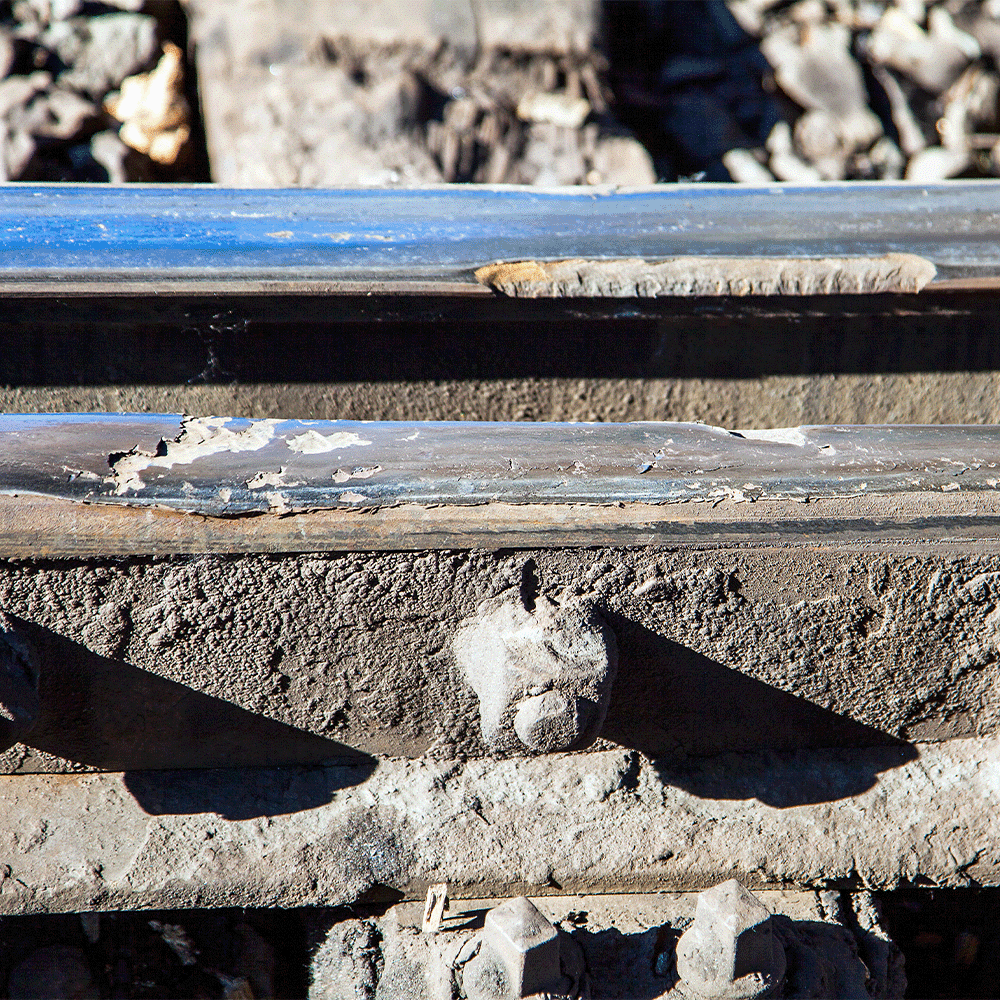
When repeated cyclic stress (rolling or sliding contact over time) is the wear mode (e.g. in bearings or rails) combined with a corrosive environment, you get a mix of fatigue crack initiation and corrosion at crack tips. This can be thought of as a subtype of tribocorrosion where the mechanical mode is primarily fatigue. Small surface cracks form from stress cycles, then corrode, then propagate further under stress. This is often termed corrosion fatigue or stress-corrosion cracking. It’s seen in things like rail wheels (contact fatigue + humidity) or turbine blades exposed to salt fog and vibration.
A cold and damp climate combined with repeated cyclic stress creates contact fatigue corrosion on these damaged train rails.
Biotribocorrosion
This refers to tribocorrosion phenomena in biological environments (human or animal body, or other living systems). The mechanisms (wear + corrosion) are similar, but the environment is typically physiological fluids, and the consequences include biological reactions. For example, a metal-on-metal hip implant undergoes wear in synovial fluid; simultaneously the fluid corrodes the metal and carries away metal ions and debris, which can cause inflammation in tissues. Biotribocorrosion is critical for medical implants (hip and knee joints, dental implants, etc.), since it affects implant longevity and biocompatibility. Even materials like Ti-6Al-4V, which are very corrosion-resistant, can wear and corrode over years of service in the body, releasing particles that may lead to osteolysis (bone loss). Research in biotribocorrosion aims to minimize these effects via better materials and coatings on implants.
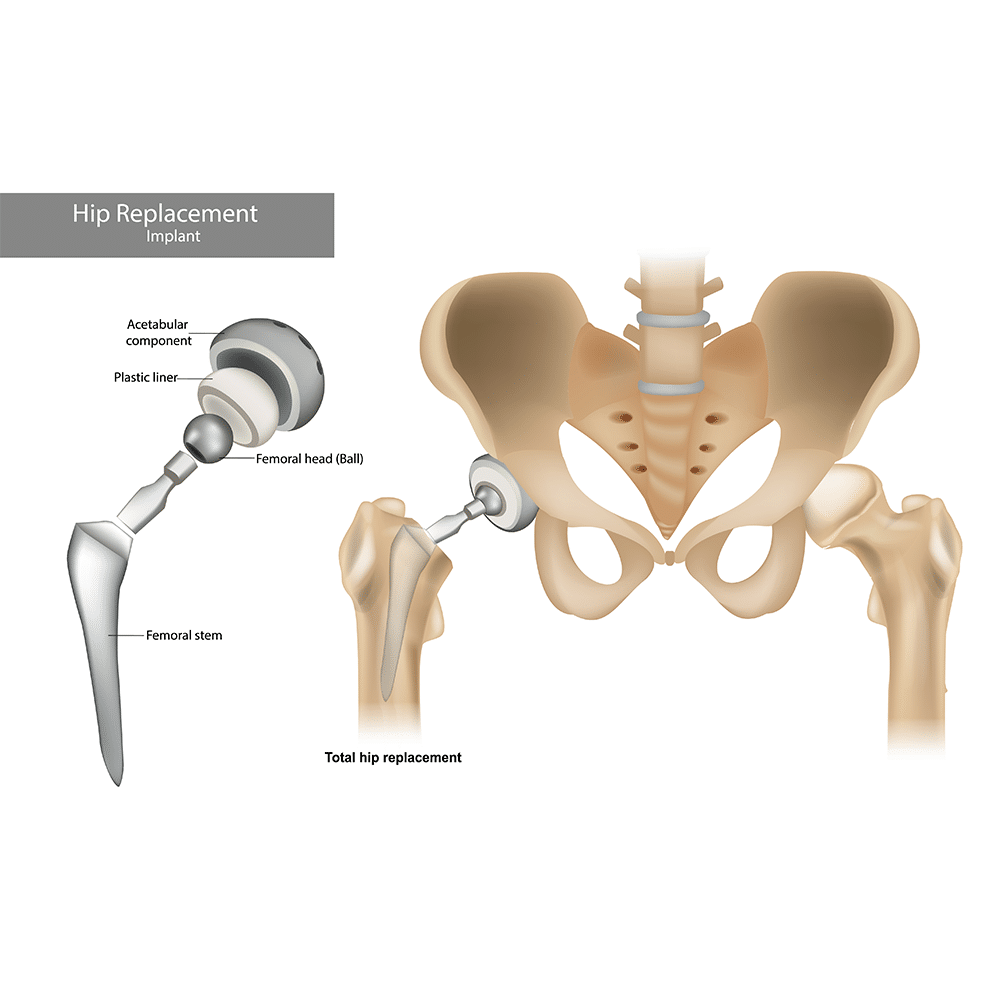
These hip replacement implants are plated advanced surface treatments to drastically reduce wear and protect from corrosion while in contact with human tissue.
(Note: Some literature may also mention “wear-accelerated corrosion” and “corrosion-accelerated wear” as descriptive terms for tribocorrosion sub-types. In practice, these overlap with the categories above. The key idea is that any mode of mechanical wear can pair with corrosion – whether it’s sliding, impact, particle erosion, or vibration – leading to a tribocorrosion scenario.)
Tribocorrosion Testing Methods
Studying tribocorrosion in a controlled way is challenging because it requires simultaneously replicating mechanical wear and corrosive conditions. Over the years, researchers have developed specialized tribocorrosion test rigs and protocols to investigate this phenomenon:
Tribometers with Integrated Electrochemical Cells
A common approach is to use a standard tribometer (for example, a pin-on-disc or reciprocating wear tester) modified to hold a liquid electrolyte and reference electrodes. The sample (metal specimen) is typically the working electrode in an electrochemical cell, and it is worn against a counter-face (pin or ball) while immersed in a corrosive solution (like NaCl). This setup allows one to control the electrochemical potential of the sample (using a potentiostat) and simultaneously measure friction and wear. By controlling or monitoring the potential/current, one can separate pure mechanical wear from corrosion-induced loss. For instance, performing a wear test under a fixed anodic potential can simulate a worst-case corrosive condition, while open-circuit (natural corrosion potential) tests show the self-driven corrosion during wear.
Measuring the current flow during sliding gives insight into instantaneous corrosion rates during rubbing events. When the current spikes, it indicates depassivation; when it drops, repassivation. Post-test, the worn surface can be analyzed for how corrosion products and wear scars develop together.
Volume Loss and Synergy Calculations
Typically, tribocorrosion tests are analyzed by conducting three variations: (1) a pure wear test in inert or non-corrosive conditions (to measure mechanical wear alone), (2) a pure corrosion test with no wear (to measure corrosion loss alone, e.g. via weight loss or charge passed), and (3) the combined tribocorrosion test (wear + corrosion). From these, one can compute the synergistic material loss using formulas given in guides like ASTM G119.
ASTM G119 (“Standard Guide for Determining Synergism Between Wear and Corrosion”) provides a framework for calculating how much extra material loss is due to interaction, beyond the sum of wear-only and corrosion-only losses. In practice, gravimetric methods (weighing samples before and after) and profilometry (3D scanning of wear scars) are used to quantify total material removal. Electrochemical data (Faraday’s law can convert charge to equivalent mass dissolved) helps quantify the portion of loss due purely to corrosion during the test. The difference between total loss and this electrochemical dissolution can be attributed to mechanical wear, and any discrepancy from additive predictions is the synergy.
Standardized Test Protocols
Researchers often follow or adapt standards such as ASTM G119 for analysis and ASTM G149 (for scratch testing in corrosive environments) or various ISO tribocorrosion standards that have emerged. Specific industries have devised tests, for example: in biomedical tribocorrosion testing of implants, protocols might involve simulated body fluid at 37 °C, with cyclic loads representative of gait, and periodically measuring metal ion release. Another example is the UNE 112086 standard (as noted in recent literature) for tribocorrosion of implant materials. While tribocorrosion doesn’t have a single universal test standard, these guides ensure researchers report comparable metrics like wear volume, corrosion current, friction coefficient, and synergy factors.
Analytical Techniques
After a tribocorrosion test, examining the surfaces and corrosion products is crucial. Microscopy (SEM, TEM) reveals wear patterns and whether corrosive pitting occurred. Surface analysis (EDS, XPS) can identify oxides or tribofilms formed. Electrochemical impedance spectroscopy (EIS) sometimes monitors how the surface film’s protectiveness changes due to wear. Such combined mechanical-electrochemical analyses help elucidate mechanisms (e.g., was material loss dominated by dissolution or by spalling of wear debris? Did the alloy form a lubricious oxide layer or did it continually corrode?). By understanding these details, one can propose ways to improve performance.
To recap, tribocorrosion testing is multifaceted: it involves simulating real service conditions as closely as possible (sliding speed, load, environment chemistry) while capturing both mechanical and chemical data. Thanks to such testing methods, engineers can compare materials (for example, seeing if a certain coating reduces tribocorrosion current and wear volume) and develop models to predict lifetime in a given environment.
Examples from Real-World Applications
Tribocorrosion is not just a laboratory curiosity – it has very tangible impacts on many real-world systems. Below are several application areas with notable tribocorrosion issues (and in some cases, benefits):
Biomedical Implants
In artificial hip joint replacements, especially metal-on-metal designs, tribocorrosion is a dominant degradation mode. The rubbing of the cobalt-chrome or titanium alloy components in body fluids leads to wear of the surfaces and corrosion of the metal, releasing debris and metal ions. This can cause inflammation in surrounding tissue and contribute to implant loosening or failure. For example, fretting corrosion at the modular head-neck junction of a hip implant can produce corrosive black debris (a mix of oxidized metal wear particles).
Tribocorrosion is also a concern for dental implants (tiny motions at the bone-implant interface in saliva can cause fretting and corrosion) and for cardiac stents or heart valves (though primarily these see corrosion, any relative motion can introduce wear). Understanding biotribocorrosion is critical to extending implant lifetime and biocompatibility. Researchers are developing hard, inert coatings (like diamond chrome) and new alloy formulations to reduce wear and corrosion in implants, as well as investigating how lubrication by synovial fluid proteins might reduce tribocorrosion.
The rounded, ball-and-socket design of this hip implant mimics natural joint motion – but this very motion also creates a natural environment for wear over time.
Marine & Offshore Equipment
Ocean environments are highly corrosive, and when you add mechanical forces from waves, currents, or machinery, tribocorrosion is inevitable. A prime example is a ship’s propeller or pump impeller made of nickel-aluminum bronze: it operates in seawater (corrosive) and is subject to cavitation and erosion from water flow (mechanical wear). This leads to rapid erosion-corrosion of the propeller blades – they develop pitting and material loss that reduce efficiency over time. Similarly, offshore oil platforms and wind turbines have components like bearings, gears, or riser pipes that undergo wear in seawater or sand-laden water.
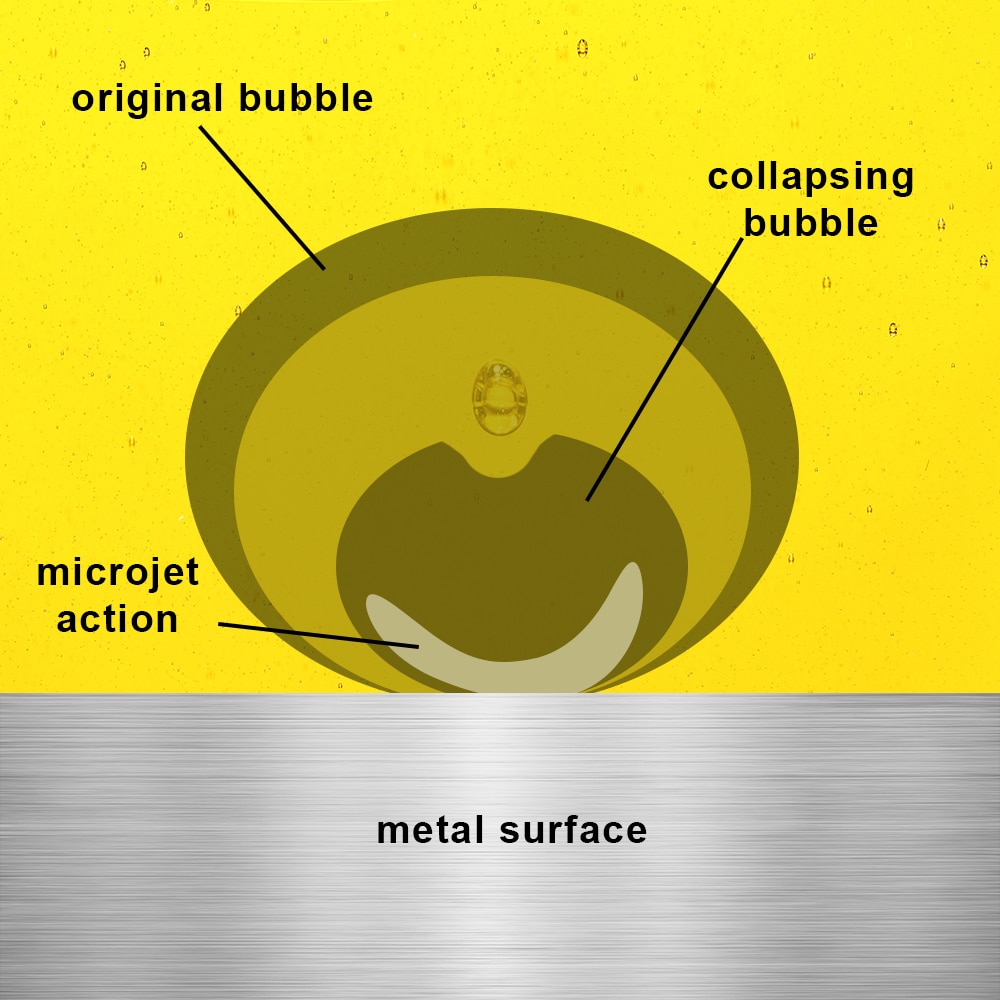
Cavitation corrosion (bubble collapse hammering the metal and causing corrosion at the impact sites) is a specific tribocorrosion threat for marine propellers and hydro-turbines. Protective measures include cathodic protection (to reduce corrosion potential) and hard coatings on surfaces to resist wear. Nonetheless, tribocorrosion remains a major limiting factor in the maintenance intervals for marine machinery.
The action of cavitation corrosion is depicted above, illustrating the collapse of a vapor bubble and microjet initiation creating a cavitation process.
Mining & Energy Industry
Many heavy industry systems face combined wear and corrosion. Mining slurry pumps and pipes are one example – they transport abrasive ore particles in water or chemicals, causing continuous abrasion of inner surfaces plus corrosive attack by the water (and sometimes acid if processes like mineral processing are involved). The result is aggressive abrasion-corrosion that can wear through thick metal pump casings if not mitigated. Waste incinerators and boilers present another case: ash particles erode tube surfaces while high-temperature corrosive gases attack the metal, a tribocorrosion-like scenario that leads to frequent tube replacements.
In the nuclear industry, a form of tribocorrosion is seen in reactor coolant systems: flow-induced vibration can cause fuel rods to fret against their supports, and the high-purity water (though not very corrosive) still contributes to corrosion of the fretted spot. This fretting corrosion of fuel rods is a safety concern. Similarly, steam turbines can experience droplet erosion (water droplet impacts in the last stages) coupled with condensate corrosion. In all these cases, tribocorrosion shortens equipment life, requiring careful material selection and protective measures (coatings, corrosion inhibitors, design changes to eliminate relative motions, etc.).
The slurry pressure pump wheel pictured above was subject to constant abrasive media flow, resulting in cavitation that further developed into deep pits.
Manufacturing & Processing
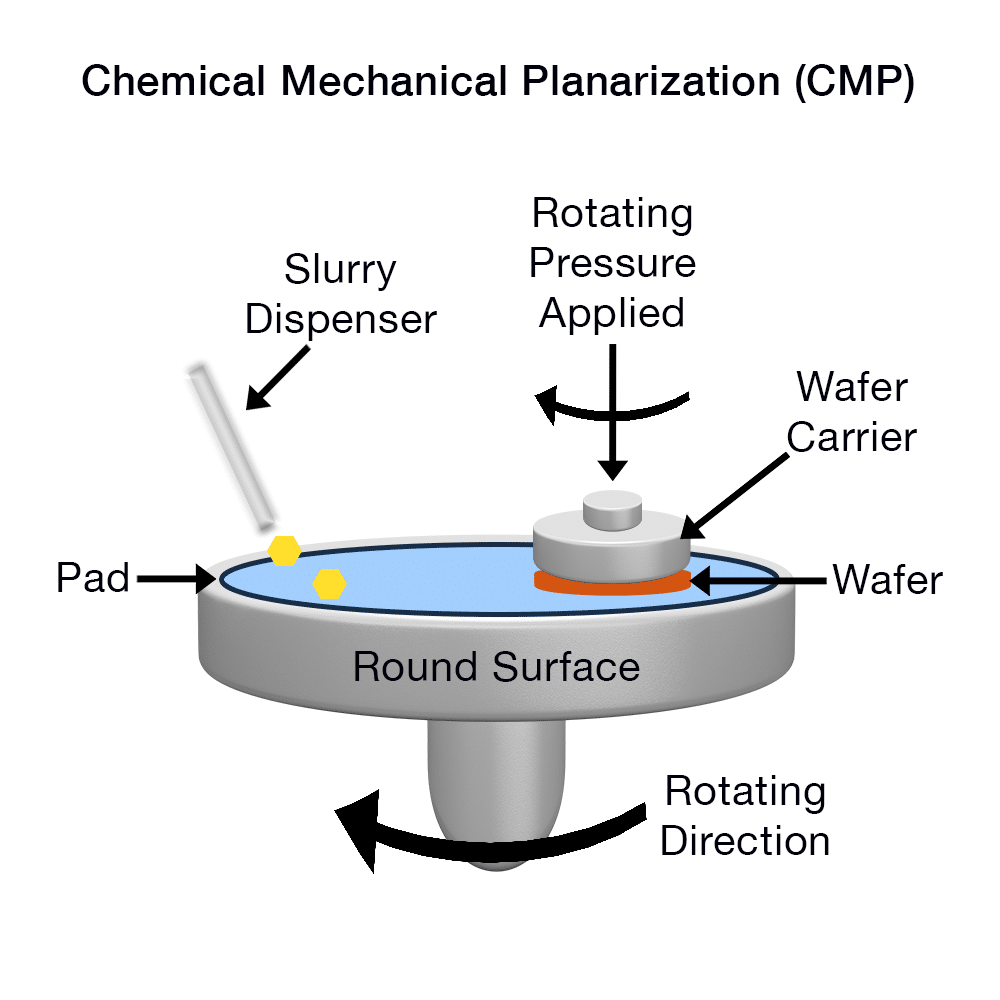
Not all tribocorrosion is detrimental; some processes harness it beneficially. Chemical-Mechanical Planarization (CMP) in the semiconductor industry is essentially tribocorrosion on purpose: a silicon wafer is pressed against a pad with abrasive particles in a reactive slurry, so that material is worn off and chemically etched simultaneously, yielding an ultra-smooth surface. Here the synergy is tuned to achieve precise polishing. Another example is machining or grinding with coolant fluids – the coolant (often water-based emulsions) provides lubrication and cooling but also can slightly corrode the freshly exposed metal, which in some cases helps remove debris or passivates the surface against further oxidation of the hot chip.
These controlled tribocorrosion processes are examples where understanding the wear-corrosion interplay is used to advantage. However, in most manufacturing contexts, uncontrolled tribocorrosion (like rust forming on a cutting tool that’s repeatedly heated and cooled, or machining titanium which forms oxides that abrade the tool) is something to avoid. Thus, even in manufacturing, engineers pay attention to using proper lubricants and corrosion inhibitors to minimize any unintended tribochemical wear.
A basic schematic of chemical mechanical planarization (CMP).
As these examples show, tribocorrosion spans diverse scenarios – from the human body to the ocean to factories. In each case, addressing tribocorrosion often requires interdisciplinary solutions: e.g., metallurgists develop more resistant alloys, tribologists improve lubrication regimes, and corrosion engineers add cathodic protection or coatings. Recognizing tribocorrosion early in the design of equipment can help in selecting the right materials and protective strategies to avoid unexpected failures in service.
Current Research Themes:
In academic and industrial research, tribocorrosion is a hot topic with several active areas of investigation:
Materials and Coatings Development
A major theme is creating alloys or coatings that can better resist the dual attack of wear and corrosion. This includes research into advanced materials like high-entropy alloys (HEAs), and novel composites that maintain protective films under wear, as well as hard coatings (ceramics, thin dense chrome (TDC), diamond-like carbon) that can act as a barrier between the metal and environment. For example, coatings on orthopedic implants or surface-hardening treatments for marine alloys are studied to see if they lower tribocorrosion rates.
Mechanistic Understanding and Modeling
Scientists are working to model tribocorrosion processes using computational tools, combining mechanical contact models with electrochemical kinetics. Multi-physics models that simulate how a sliding contact evolves with simultaneous oxidation are helping predict material loss over time. There’s also interest in the fundamental mechanisms at the microscale – e.g., using in-situ techniques to watch a passive film break and regrow, or to measure the transient currents during a single fretting event. The goal is to build predictive models that can inform design without always needing long and expensive experiments.
Standardization of Testing and Protocol
Given the complexity of tribocorrosion testing, researchers are striving to establish standard test methods so that results are comparable. As mentioned, ASTM G119 gives a framework for synergy calculation, but there’s ongoing development of standardized tribocorrosion tests for specific applications (like standardized implant wear-corrosion tests). Round-robin tests between laboratories and new techniques (like zero-resistance ammetry for measuring fretting corrosion currents) are being evaluated. A more standardized approach will help engineers reliably rank materials for tribocorrosion resistance.
Application-Specific Studies
Much tribocorrosion research is targeted at particular industries. Biotribocorrosion of medical devices is one of the leading areas – with studies mapping how different alloys corrode-wear in simulated body fluid, or how implant design changes (e.g. modular vs monoblock implants) affect tribocorrosion outcomes. In the marine field, researchers examine coatings and cathodic protection to reduce propeller erosion-corrosion. In energy, there are efforts to mitigate fretting in nuclear reactors by material tweaks or better supports. Sharing knowledge across these domains is a challenge, so there are conferences and journals now specifically dedicated to tribocorrosion and bio-tribocorrosion to disseminate findings.
Preventative and Monitoring Strategies
Finally, an important practical theme is developing ways to detect and prevent tribocorrosion in service. For example, using acoustic emission sensors or electrochemical noise to detect the onset of tribocorrosion in a machine before it fails, or designing smart coatings that self-heal their passive layer after scratching. Lubricant chemistry is also being tuned (for instance, adding corrosion inhibitors in lubricants) to see if it can suppress corrosion during boundary lubrication. The intersection of tribocorrosion with fields like nanotechnology (e.g., adding nanoparticles in lubricants to form protective tribofilms) is an emerging cross-disciplinary trend.
Overall, tribocorrosion research is tackling the “how and why” at a fundamental level – how do wear and corrosion specifically interact for a given material, and why does that lead to failure – as well as the “what can we do about it” at an engineering level – designing better materials, coatings, and maintenance strategies. It’s a rapidly evolving field, reflecting its importance in ensuring reliability of everything from implants to industrial machinery.