What is Uniform Corrosion?
Uniform corrosion is characterized by the development of corrosion products across the entire surface of a material or part. Unlike some other forms of corrosion, where certain localized areas are more prone to corrosive metal failure, in uniform corrosion, no specific spot is more susceptible than another; instead, corrosion occurs uniformly across the surface, affecting all areas equally.
In this post — the third in our series on metal failure mitigation — we’ll provide an overview of uniform corrosion, where it is most likely to occur, and how to mitigate the effects using Armoloy TDC (Thin Dense Chromium).
Where Does Uniform Corrosion Occur?
Uniform or “general” corrosion primarily affects steel more than any other material. Steel components exposed to outdoor environments, particularly those with high levels of salt and humidity in the air, are especially susceptible to uniform corrosion. Salty, humid air exacerbates the corrosion process, leading to accelerated degradation of steel surfaces.
While corrosion most commonly affects steel, copper is also susceptible to uniform corrosion. However, the green oxide layer on copper surfaces acts as a protective barrier, often considered desirable for its aesthetic appeal and corrosion-resistant properties.
In contrast, metals with inherent corrosion resistance, such as aluminum and stainless steel, are less prone to uniform corrosion. These metals form passive oxide layers that protect them from environmental degradation, mitigating the risk of uniform corrosion in most outdoor environments.
Components Susceptible to Uniform Corrosion
The components and assemblies susceptible to uniform corrosion are typically exposed to outdoor environments or involved in fluid handling operations, such as pumps, valves, pipes, and tanks.
- Pumps, which move fluids in industrial and commercial applications, are often made of metals susceptible to corrosion, such as steel or cast iron.
- Valves, used to control the flow of fluids, are also vulnerable to corrosion, particularly in regions with higher levels of atmospheric pollutants or where corrosive fluids are handled.
- Pipes, which transport water, chemicals, or gases, are constantly exposed to the elements and are prone to uniform corrosion over time, especially at joints and bends where the protective coating may be compromised.
- Tanks that store various liquids or gases also experience uniform corrosion, particularly along exposed surfaces.
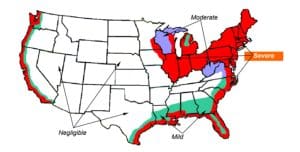
As seen below, uniform corrosion is most likely to occur along the coastlines and in heavily industrialized areas, such as the aptly named “rust belt.” These regions experience higher levels of atmospheric pollutants, salt in the air from ocean spray, and exposure to industrial chemicals, all contributing to accelerated corrosion rates.
Figure 1. Map showing areas of severe, moderate, and mild corrosion in an outdoor environment.
Testing Methods for Uniform Corrosion
Corrosion rate, often expressed in terms of mass loss per year (mg/yr) or thickness loss per year (mils/yr), is a crucial parameter for assessing the degradation of materials due to corrosion. Accurately measuring corrosion rates is essential for evaluating the performance and durability of materials in various environments.
Reproducing real-world corrosion rates in laboratory settings poses significant challenges. While laboratory tests aim to simulate environmental conditions, they often fail to fully replicate the complex interactions and variability observed in actual outdoor exposure. Achieving accurate and reliable results requires careful design and execution of corrosion testing protocols.
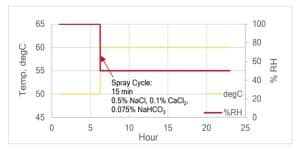
Figure 2. Typical test profile for SAE J2334 Cyclic Corrosion Test.
Cyclic corrosion testing has emerged as one of the most successful methods for simulating real-world corrosion conditions in the laboratory. This approach involves subjecting test specimens to multiple exposure cycles, including temperature, humidity, wetting, and drying periods. One example of cyclic corrosion testing is the SAE J2334 standard, commonly used in the automotive industry, which accelerates corrosion rates to simulate years of exposure in a relatively short time frame.
Outdoor exposure testing entails placing test specimens in racks outdoors to assess their corrosion performance under real environmental conditions. While this method provides valuable insights into real-world corrosion behavior, it lacks acceleration factors, meaning tests may take several years to generate meaningful data. Despite this limitation, outdoor exposure testing remains a valuable tool for evaluating long-term corrosion resistance.
For corrosion testing of industrial equipment operating in corrosive environments (such as pipes and pumps), ASTM has published guidelines to help develop rigorous, repeatable testing procedures. ASTM G32 provides recommendations for designing laboratory tests tailored to specific industrial applications, ensuring consistent and reliable assessment of corrosion resistance.
Uniform Corrosion Mitigation Strategies
Several strategies can mitigate uniform corrosion, including material selection, galvanizing, anodic protection, and organic barrier coatings like paints and epoxies.
Material selection plays a vital role, with aluminum, stainless steel, and weathering steels offering good resistance to corrosion in various environments. Galvanizing, especially hot dip galvanizing (HDG), can slow uniform corrosion by sacrificially corroding a zinc coating while protecting the base metal. Additionally, anodic protection is employable, utilizing sacrificial zinc or magnesium anodes, although regular replacement of these anodes is necessary.
Organic barrier coatings, such as paints and epoxies, are common choices to protect against uniform corrosion. While they provide good protection for many years, they are susceptible to damage. For example, auto body panels often use a combination of galvanizing and multiple organic topcoats to achieve excellent corrosion resistance.
Armoloy TDC (Thin Dense Chromium) can also improve resistance to corrosion and is particularly beneficial in applications requiring wear resistance, cleanability, or release properties, areas where organic coatings may fall short. For instance, in valve bodies subject to corrosive fluids and some erosion or in food processing equipment needing corrosion resistance while remaining easy to clean, Armoloy TDC can offer significant advantages. Armoloy Processing Engineering Center experts have a commitment to deliver top-tier results at the least possible cost.