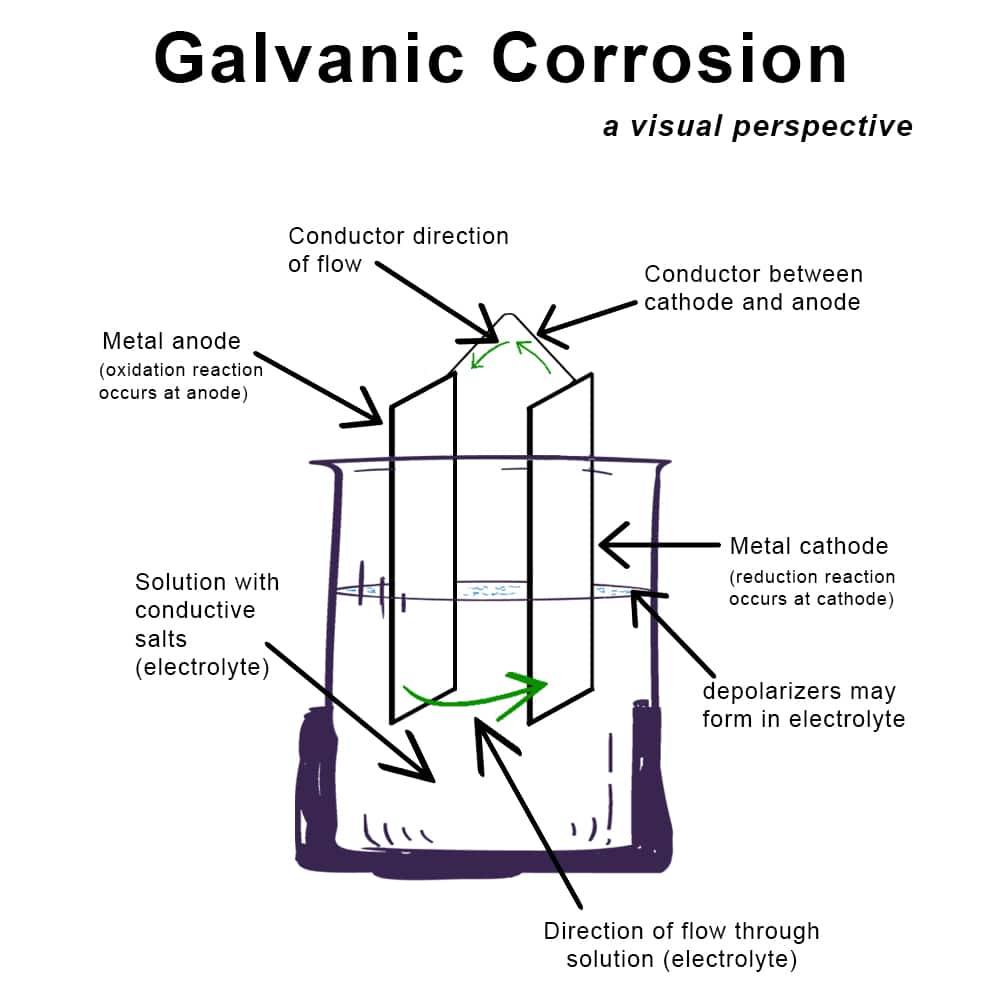
Galvanic corrosion occurs when two dissimilar metals are in electrical contact in the presence of an electrolyte. Here’s a list of common metals and their propensity to cause galvanic corrosion when paired with other metals:
- Zinc: Highly anodic and likely to corrode when in contact with more noble metals like copper or stainless steel.
- Aluminum: Anodic, corrodes when paired with metals like stainless steel, copper, or brass.
- Steel (Carbon and Low Alloy): Intermediate, can corrode when in contact with more noble metals like copper, bronze, or stainless steel.
- Copper: Cathodic, causes corrosion in more anodic metals like zinc, aluminum, or galvanized steel.
- Brass/Bronze: Cathodic, leads to corrosion in anodic metals such as aluminum or steel.
- Stainless Steel: Highly cathodic, can cause significant corrosion in anodic metals like zinc, aluminum, or regular steel.
Galvanic Series: The tendency of metals to cause galvanic corrosion can be predicted using the galvanic series, which ranks metals from most anodic (active) to most cathodic (noble). Metals that are far apart in this series are more likely to cause galvanic corrosion when in contact.
Preventive Measures:
- Avoid Direct Contact: Use insulating materials to separate dissimilar metals.
- Coatings: Apply protective coatings to more noble metals.
- Material Compatibility: Choose metals close together in the galvanic series to reduce potential differences.
By understanding which metals are likely to cause galvanic corrosion, you can make informed material choices and implement preventive strategies to protect your components.