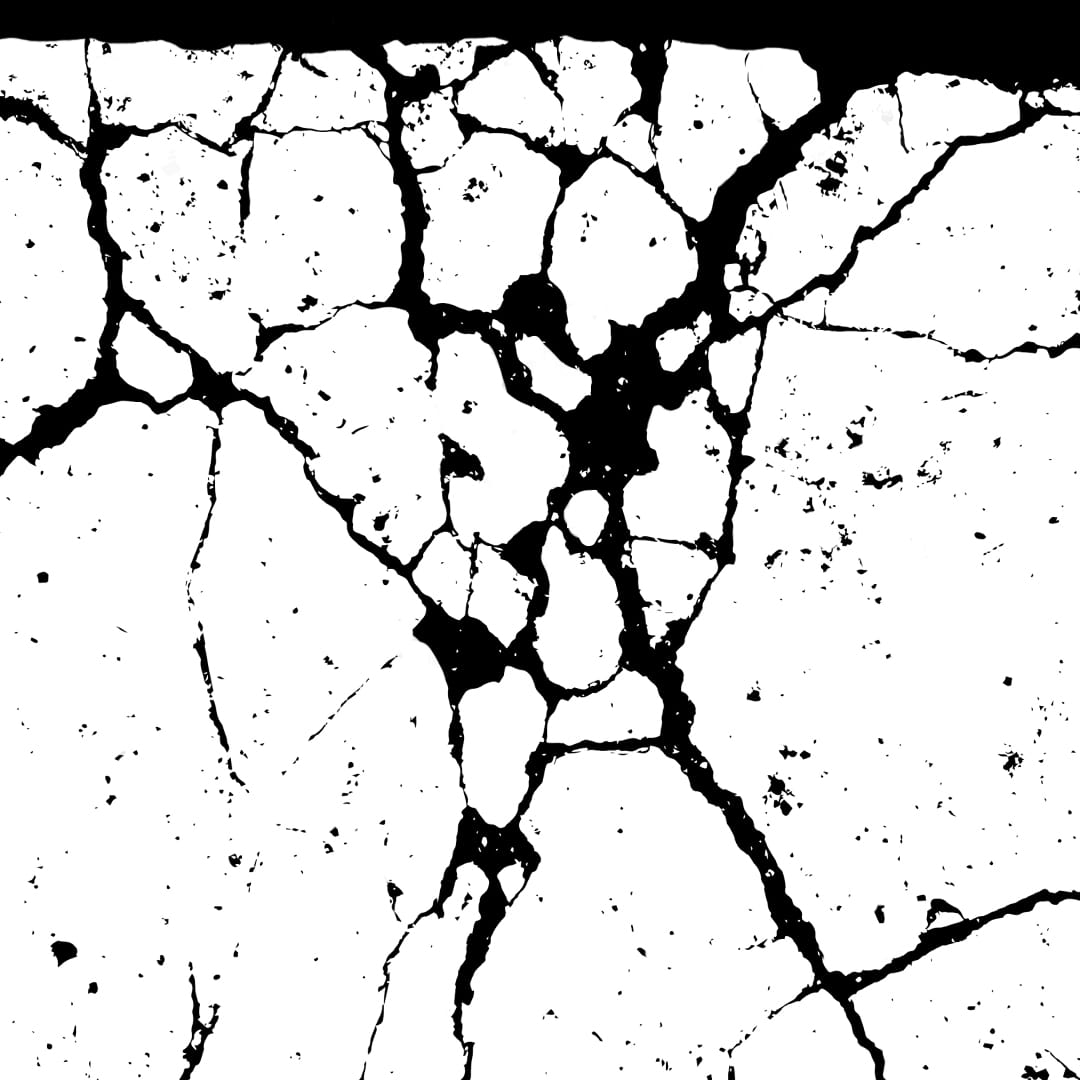
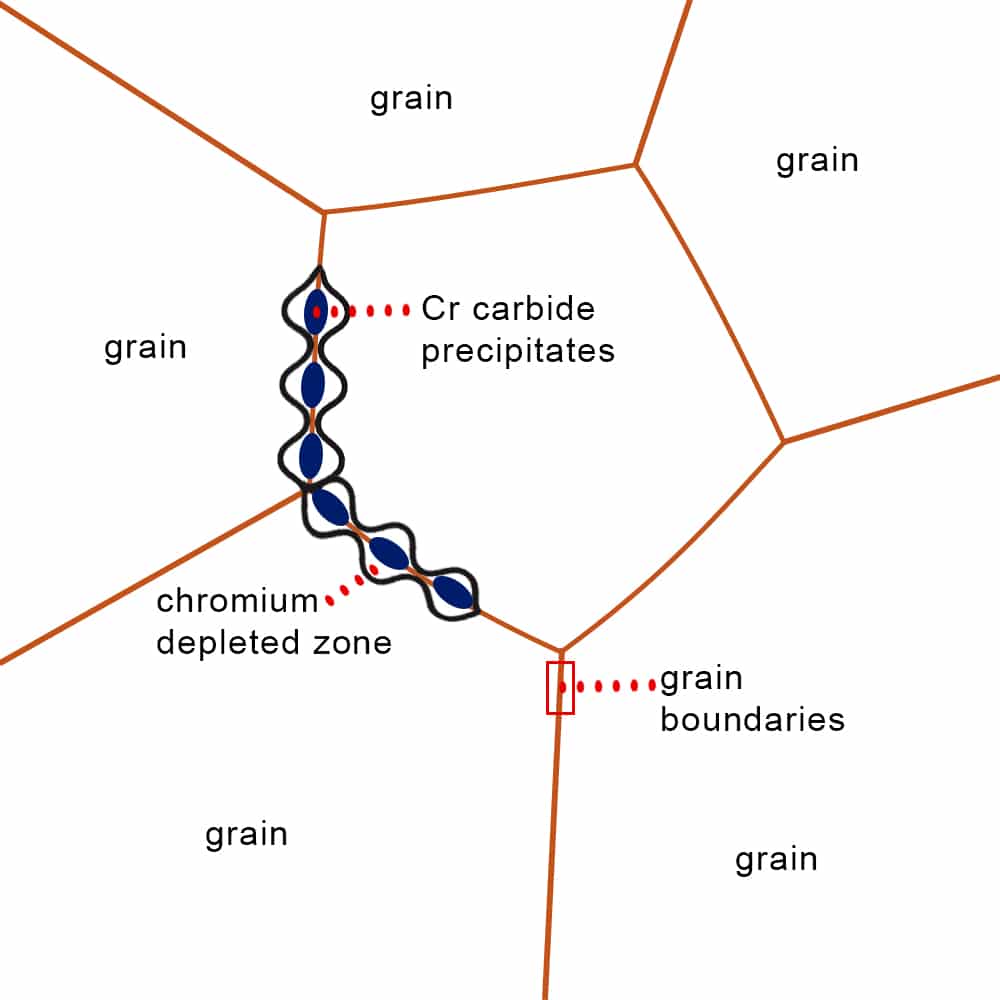
Intergranular corrosion in aluminum occurs when corrosion selectively attacks the metal along its grain boundaries rather than uniformly across the surface. This form of localized corrosion is often triggered by a combination of alloy composition and thermal processing.
- Heat Treatment: Improper or excessive heat treatment can cause alloying elements like magnesium or silicon to precipitate along grain boundaries, creating weak points for corrosion to start.
- Alloy Composition: Aluminum alloys containing copper or other reactive elements are more prone to intergranular corrosion due to the formation of electrochemically active zones at grain edges.
- Environmental Exposure: Chloride-rich or high-humidity environments can accelerate this type of attack, especially in marine or industrial settings.
- Galvanic Activity: A difference in electrochemical potential between the grain boundary and surrounding metal creates a galvanic cell that drives localized corrosion along those boundaries.
How to prevent intergranular corrosion:
- Controlled Heat Treatment: Use precise thermal processes to avoid sensitization and harmful phase formation at grain boundaries.
- Alloy Selection: Choose aluminum alloys with lower susceptibility — such as those with balanced compositions and minimal copper content.
- Protective Coatings: Apply anodizing or corrosion-resistant coatings to provide a barrier against environmental exposure.
Proper alloy design, heat control, and surface protection can significantly reduce the risk of intergranular corrosion in aluminum components.