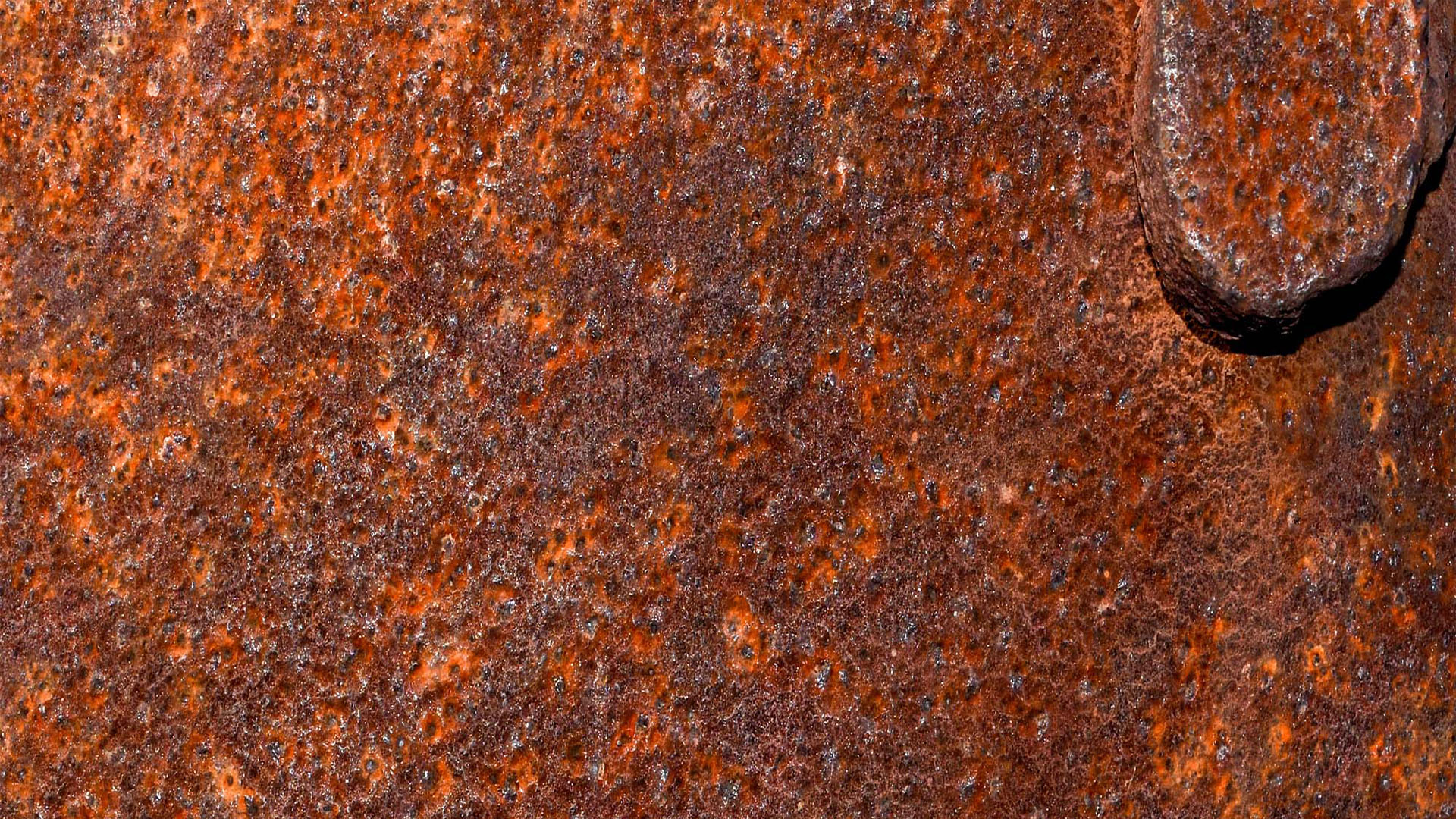Uniform corrosion refers to a widespread, even attack across the surface of a metal — and rust is a classic example of this type of degradation. When iron or steel is exposed to moisture and oxygen, it reacts to form iron oxide, or rust, which develops fairly evenly over exposed areas.
While the corrosion may look patchy due to environmental conditions, the chemical process occurs consistently across the entire surface. Over time, this leads to a gradual and uniform thinning of the metal, which can weaken structural components if left untreated.
Rust is most common in outdoor steel structures, unprotected tools, and automotive components exposed to rain or humidity. It’s typically slower than localized corrosion but still dangerous due to its broad impact.
Preventive strategies include:
- Protective Coatings like thin dense chrome, paint, or galvanization
- Environmental control to reduce exposure to water and oxygen
- Use of corrosion-resistant alloys such as stainless steel or weathering steel
Learn more about the types of corrosion and how they differ at 5 Types of Corrosive Metal Failure.