Armoloy Accreditations
What is ISO 10993
ISO 10993 is the international standard for assessing whether medical devices are biologically safe for human contact. This includes the materials used in medical devices, such as metal coatings and surface treatments. At Armoloy, ISO 10993 improves the safety and performance of surgical tools, implants, and other components that come into contact with the body.
Developed by the International Organization for Standardization (ISO), this standard categorizes medical devices based on how long and in what way they interact with the human body.
It assesses risks like cytotoxicity (cell damage), sensitization (allergic reactions), and systemic toxicity (harm to the whole body).
Manufacturers rely on ISO 10993 biocompatibility testing to prove their devices meet biological safety requirements. This is a critical step in gaining regulatory approval, ensuring patient safety, and earning the trust of healthcare providers and medical industry stakeholders worldwide.
Key Principles of ISO 10993 Biocompatibility Testing
ISO 10993 addresses five core principles for testing the biocompatibility of medical devices:
- Establish a universal benchmark for biocompatibility testing to ensure coated materials are safe for human contact.
- Protect patient safety with strict testing criteria to prevent cytotoxicity, sensitization, and other adverse biological effects.
- Streamline regulatory approvals by demonstrating that coatings on implants, surgical tools, and orthopedic devices meet global biocompatibility and safety standards.
- Enhance device performance with coatings that improve corrosion resistance, wear protection, and longevity without compromising biocompatibility.
- Build industry trust by guaranteeing that biocompatible coatings meet the highest quality and safety standards.
ISO 10993 compliance is essential for medical device coatings, helping manufacturers deliver safe, high-performance solutions for the healthcare industry.
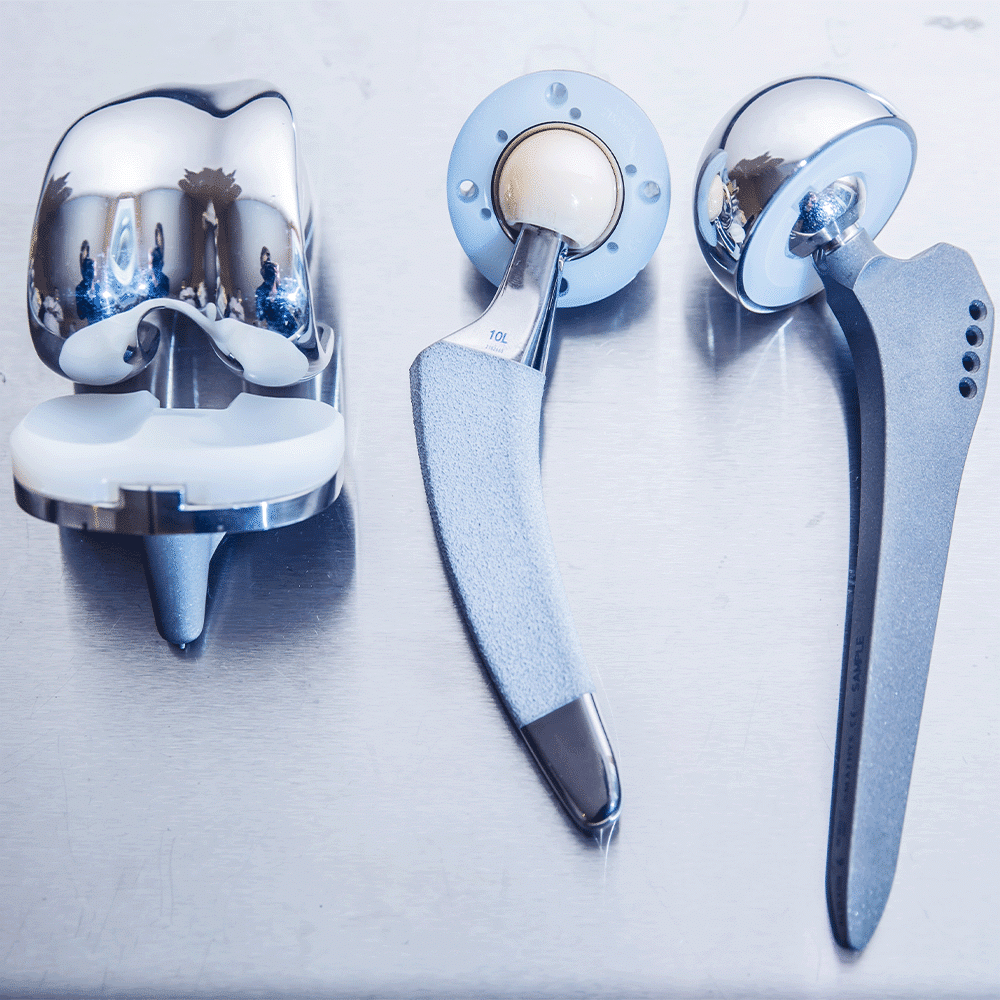
ISO 10993 Biocompatibility Testing Requirements
Adhering to ISO 10993 for medical device coatings involves a clear, structured process:
- Identify Contact Type: Identify where and how the device contacts the body—skin, blood, or tissue—to determine required biocompatibility tests.
- Assess Biological Risks: Evaluate the coating’s materials, application, and intended use to identify potential biological hazards.
- Analyze Material Composition: Examine the chemical makeup of the coating and any potential leachables to understand its interaction with biological systems.
- Select Tests: Based on the risk assessment and device application, choose the proper biocompatibility tests, such as cytotoxicity, sensitization, or irritation.
- Evaluate Results: Review the test data to confirm the coated device meets ISO 10993 biocompatibility standards.
This process ensures that coated medical devices comply with strict biological safety requirements, protecting patients and supporting regulatory approval worldwide.
ISO 10993 Applications Across the Medical Industry
ISO 10993 ensures the biocompatibility of medical devices across diverse sectors, including medical device manufacturing, healthcare, pharmaceutical, and biotechnology. Key applications include:
- Implantable Devices: Pacemakers, stents, orthopedic implants, and other devices placed inside the body.
- Surgical Instruments: Scalpels, forceps, endoscopic tools, and other instruments used in surgical procedures.
- External Devices: Bandages, catheters, and other devices that come into contact with the patient’s skin or mucous membranes.
- Diagnostic Equipment: Blood glucose monitors, blood collection devices, testing kits, and other diagnostic tools.
- Drug Delivery Systems: Syringes, inhalers, infusion pumps, and other devices used to administer medications.
- Biosensors & Wearables: Devices used for health monitoring and diagnostics.
While its primary focus is on medical devices, biocompatibility principles are also relevant to other industry applications where materials interact with the human body, such as cosmetics and food packaging.

Benefits of Working with an ISO 10993 Compliant Provider
Regulatory compliance
Meets global medical device standards, streamlining approval processes
Patient safety
Identifies and reduces the risk of adverse reactions to medical devices
Proven biocompatibility
Coatings undergo rigorous evaluation to ensure safe body contact
Reliable performance
Maintains coating integrity under medical-grade conditions
Risk management
Minimizes liability by adhering to strict biological safety requirements
Market access
Facilitates entry into regulated markets like the FDA and EU MDR
Consistent quality
Standardized processes deliver consistent, high-quality results
Industry trust
Demonstrates commitment to safety and reliability, earning trust from healthcare providers and patients

Accreditations at Armoloy
Armoloy offers plating solutions that meet ISO 10993 to support both regulatory compliance and individual project needs. Explore common specifications and accreditations we work with, and contact us to find the right solution for your application.
Disclaimer: The interpretations and overviews of ISO 10993 provided by The Armoloy Corporation are solely the views of Armoloy and may not represent the views of other entities. These interpretations are intended for informational purposes only and should not be considered as definitive or legally binding. The Armoloy Corporation is not responsible for the accuracy of these interpretations and expressly disclaims any liability for loss or damage arising from their use. This disclaimer is subject to change and does not create any express or implied warranty regarding the information provided.