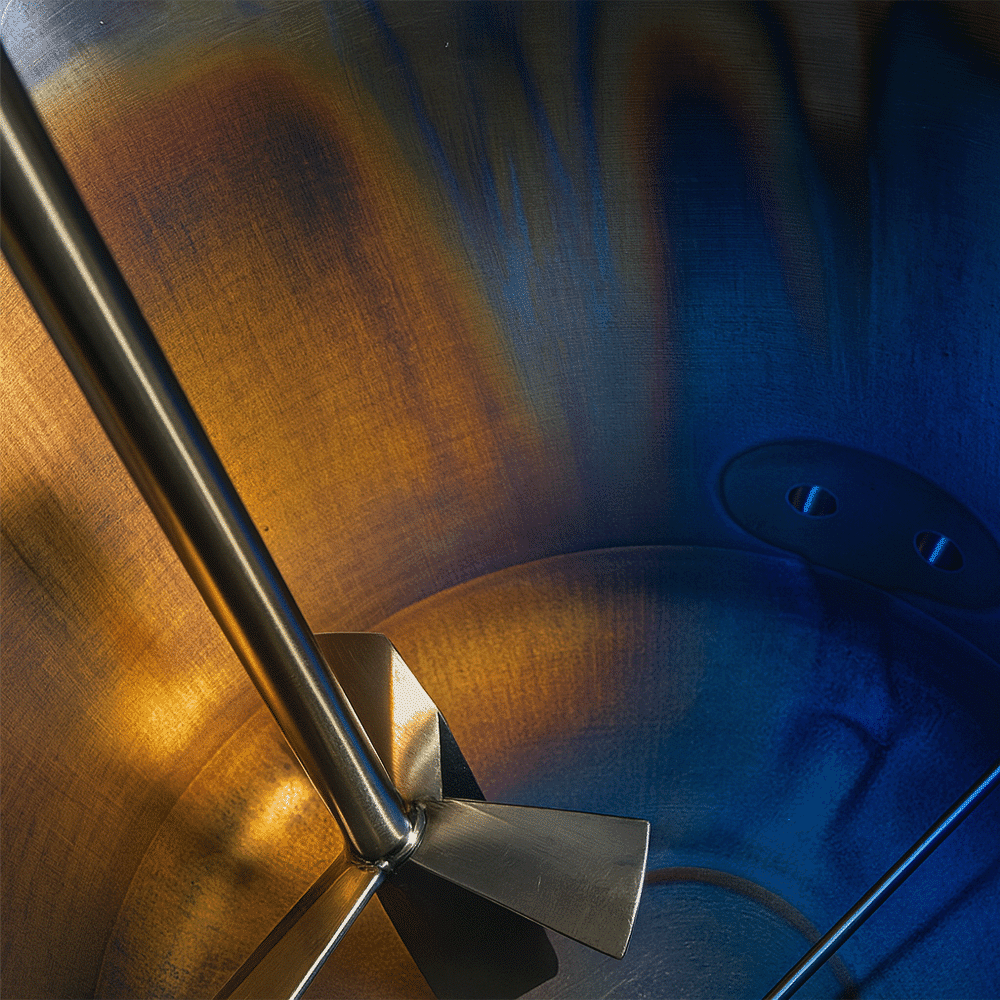
Metal Rouging in Stainless Steel: An Engineering Overview
Metal rouging is a surface discoloration phenomenon affecting stainless steel systems in pharmaceutical, food processing, semiconductor, and energy industries—especially those exposed to high-purity water or clean steam. Although often cosmetic, rouging can signal deeper changes to the metal surface. In high-purity systems, this may result in performance degradation or contamination risks.
This article outlines the mechanisms behind metal rouging, the classification of its forms, the operational environments in which it occurs, and practical approaches to mitigation.
What is Metal Rouging?
Metal rouging involves the formation of iron oxide films on stainless steel surfaces. Although stainless steel is corrosion-resistant due to its passive chromium oxide layer, under specific conditions—typically involving elevated temperature, pressure, or aggressive media—this layer can degrade or allow the diffusion of iron atoms to the surface.
The result is a discolored layer, ranging from red-orange to black or blue, depending on the oxidation state and environmental conditions.

Classification of Rouging Types
Experts typically classify metal rouging into three types—Type I, Type II, and Type III—based on their color, source of iron contamination, and the operational conditions under which they form. Understanding these distinctions is critical for selecting proper mitigation strategies and identifying root causes in stainless steel systems.
| Type | Color | Underlying Cause | Common System Conditions |
|---|---|---|---|
| Type I | Reddish or rust-colored |
External iron contamination from tools or fabrication debris | Ambient or cold stainless steel systems after fabrication |
| Type II | Dark brown or black |
Iron migration from beneath the surface due to passive layer breakdown | High-purity hot water systems such as WFI or clean steam |
| Type III | Blue, purple, or black |
Chromium depletion and internal oxidation of the metal matrix | High-temperature, oxidizing environments (e.g., steam systems) |
Proper identification of rouging types helps guide material selection, cleaning methods, and long-term corrosion control strategies for stainless steel systems.
Where Metal Rouging Occurs
Metal rouging is most commonly observed in high-purity, high-temperature systems across industries that demand sterile or ultra-clean environments:
-
- Pharmaceutical and biotech water systems: Especially in WFI (Water for Injection) and clean steam applications, where even minor discoloration can jeopardize sterility and compliance.

WFI high purity water equipment used for pharmaceutical use
- Food and beverage processing lines: Exposure to frequent clean-in-place (CIP) cycles and acidic solutions can destabilize passive layers and promote rouging.
- Semiconductor rinse and process piping: Ultra-pure water systems in chip fabrication facilities are highly susceptible to rouge due to their oxygen-depleted environments.
- High-purity utility systems in power and energy facilities: Rouging may form in steam condensate and boiler feed systems operating at elevated temperatures.
In all of these environments, stainless steel systems operate under conditions that challenge the stability of the protective chromium oxide layer—making them prime targets for rouge formation.
Risks and Consequences
While metal rouging may appear cosmetic, it can lead to functional, regulatory, and financial consequences that compromise system performance and reliability:
- Product contamination: Dislodged rouge particles or dissolved iron can compromise the purity of critical fluids in pharmaceutical, semiconductor, or food-grade systems.
- Regulatory concerns: Visible rouge deposits can raise red flags during GMP inspections, potentially delaying production or triggering remediation protocols.
- Surface degradation: Advanced stages of rouging—especially Type III—can roughen the steel surface or contribute to pitting and localized corrosion over time.
- Maintenance costs: Persistent rouging often necessitates repeated cleaning cycles, chemical passivation, or eventual material replacement.
Understanding these risks is key to implementing proactive surface treatment and monitoring strategies in stainless steel infrastructure.
Mechanisms Behind Rouging
Rouging arises from a combination of surface chemistry changes, environmental conditions, and operational stressors. The phenomenon is driven by subtle but critical breakdowns in the stainless steel’s passive protection mechanisms.
Loss or thinning of the chromium-rich passive layer is the most common trigger. This passive film, made of chromium oxide, normally prevents oxidation. However, aggressive cleaning agents, low pH solutions, or high temperatures can compromise it—exposing iron beneath the surface to oxygen and moisture.
Free iron on the surface, often introduced during fabrication or welding, acts as a corrosion site. These iron particles oxidize rapidly and serve as nucleation points for rouging, particularly in Type I cases.
Low oxygen levels in high-purity systems can ironically accelerate rouging. In ultrapure water and steam environments, the lack of oxygen hinders natural repassivation—the stainless steel’s ability to heal its protective layer—allowing iron to diffuse toward the surface unchecked.
Thermal cycling and prolonged high-temperature exposure weaken the passive layer over time. In steam systems or reactors, constant expansion and contraction can create microfractures, making it easier for oxygen and contaminants to penetrate the surface and form iron oxides.
Together, these conditions allow iron atoms from within the stainless steel to migrate outward, oxidize, and form adherent iron oxide films—creating the visible discoloration known as metal rouging.
Mitigation Strategies
Addressing metal rouging requires a multi-faceted approach that combines material selection, surface preparation, system engineering, and regular maintenance. Each strategy helps prevent the conditions that allow iron oxide films to form and persist on stainless steel surfaces.
Surface Preparation and Passivation
Mechanical finishing techniques such as electropolishing or fine grinding reduce surface roughness, which in turn minimizes the retention of contaminants and free iron particles. These smoother surfaces are less prone to rouge initiation. After fabrication, chemical passivation—typically using nitric or citric acid—restores the stainless steel’s protective chromium oxide layer, reinforcing corrosion resistance in high-purity environments.
Material Selection
Using molybdenum-enriched austenitic stainless steels like 316L offers greater resistance to rouging than standard 304 grades. In particularly aggressive or high-temperature environments, corrosion-resistant alloys such as AL-6XN or Hastelloy® may be necessary to prevent iron migration and passive layer breakdown.
System Design
Thoughtful piping and system design can significantly reduce rouging. Maintaining turbulent flow and ensuring adequate oxygen content in feed water help stabilize the passive layer. Designers should also avoid dead legs, crevices, or low-flow areas where localized corrosion can accelerate rouge formation.
Surface Coatings
In select applications, applying inert barrier coatings—such as metallic overlays or ceramic films—can effectively isolate the stainless steel substrate from aggressive media. These coatings suppress iron ion diffusion and improve thermal and chemical resistance, particularly in steam-heavy or high-purity systems.
Routine Cleaning and Inspection
Proactive maintenance is critical for early detection and mitigation of rouging. Scheduled visual inspections and validated cleaning protocols using citric acid, hydrogen peroxide, or other oxidizing agents help remove initial discoloration before it progresses. In heavily regulated environments like pharmaceutical manufacturing, routine testing and documentation also support GMP compliance.
Although metal rouging may seem like a cosmetic issue at first, it can be an early warning sign of more serious corrosion or system imbalance. By applying preventive design practices, selecting the right materials, and implementing robust surface engineering strategies, operators can reduce the frequency, severity, and operational impact of rouging across critical stainless steel systems.