
What is Pitting Corrosion?
Pitting corrosion is a localized and highly aggressive form of material degradation that manifests as small cavities or “pits” on metal surfaces. Unlike uniform corrosion, which progresses at a consistent rate across an entire surface, pitting corrosion occurs in isolated locations, making it particularly dangerous due to its ability to weaken structures while leaving much of the surface seemingly intact. This unpredictable and often undetectable form of corrosion can lead to premature failure of metal components in critical industries such as aerospace, automotive, medical devices, oil and gas, and manufacturing.
Understanding the electrochemical mechanisms, contributing factors, and effective mitigation techniques is crucial for industries reliant on corrosion-resistant materials.
This guide explores:
- Fundamentals & forms of pitting corrosion
- How surface area affects pitting
- Additional contributing causes
- Effects on various materials
- Detection methods
- Best practices for prevention
Fundamentals of Pitting Corrosion
Pitting corrosion occurs when localized anodic sites on a metal surface experience accelerated dissolution while the surrounding metal remains largely unaffected. This electrochemical reaction is typically facilitated by environmental aggressors such as chloride ions, which compromise protective oxide films and induce localized breakdown.
What makes pitting corrosion particularly hazardous is its potential for rapid propagation beneath the surface, leading to structural instability while maintaining an outwardly intact appearance. In engineering applications, pitting corrosion is a significant concern because even minor pits can serve as initiation points for fatigue failure or stress corrosion cracking. Left undetected, pitting can lead to extensive material loss, reducing load-bearing capacity and leading to catastrophic failures in structural components.
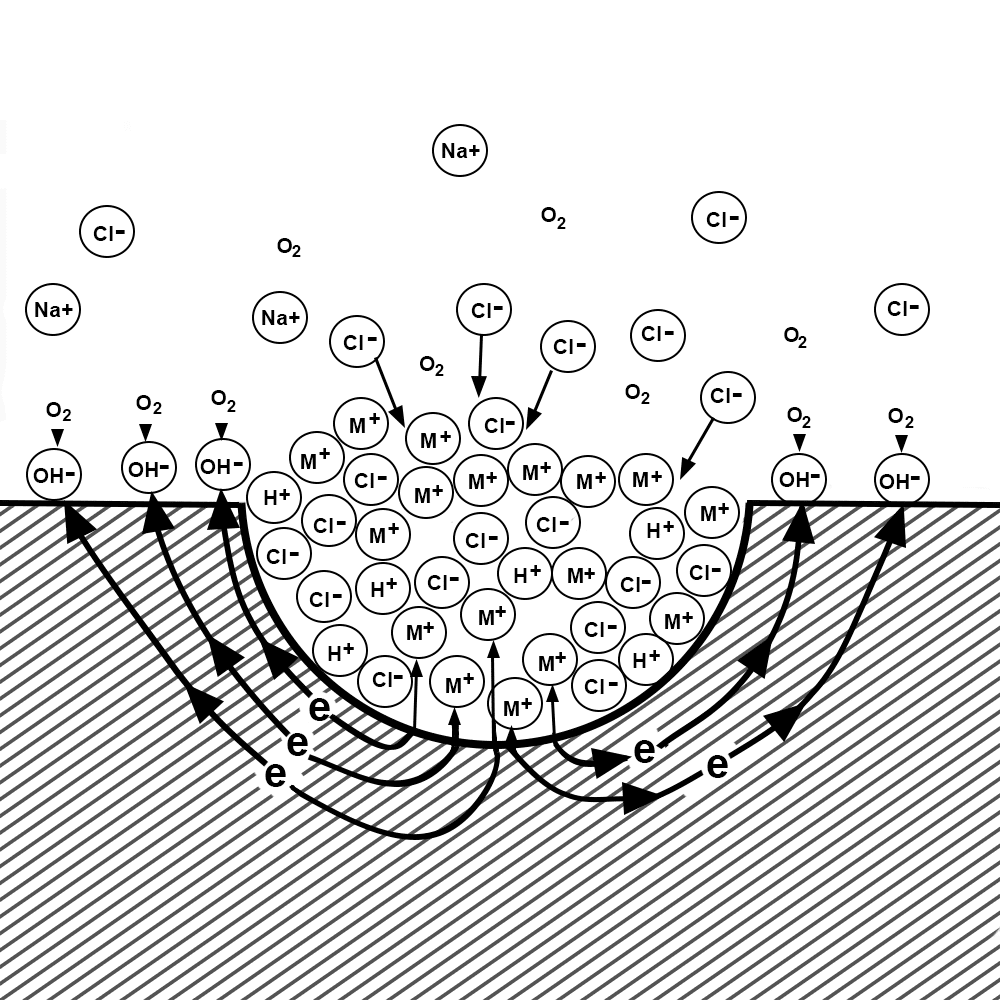
The shape of the pit itself causes corrosive species to concentrate inside the pit, accelerating the corrosion process.
Forms of Pitting Corrosion Explained
Pitting corrosion manifests in various forms, each characterized by the shape and orientation of the pits. Understanding these types is essential for accurate identification. Here are the common forms of pitting corrosion:
Narrow, Deep Pits
These pits are characterized by a small surface opening that extends deeply into the material. This geometry can lead to significant structural weakness, as the deep penetration may compromise the integrity of the component. Such pits are often challenging to detect due to their limited surface visibility.
Wide, Shallow Pits
Featuring a broad surface area with minimal depth, wide and shallow pits result in more uniform material loss. While they may not immediately threaten structural integrity, if widespread, they can lead to substantial material degradation over time.
Elliptical Pits
Elliptical pits are characterized by their elongated, oval-shaped depressions on metal surfaces. Unlike narrow, deep pits or wide, shallow pits, elliptical pits have a distinct geometry that can influence how they propagate and affect the structural integrity of materials.
Subsurface Pits
These pits initiate beneath the material’s surface, often leaving the exterior appearance largely unaffected. This hidden nature makes them particularly insidious, as they can progress undetected, leading to sudden failures.
Undercutting Pits
Undercutting pits expand laterally beneath the material’s surface, creating an overhanging lip. This form of pitting can severely undermine the material’s structural integrity, as the surface may appear intact while significant damage occurs underneath.
Vertical Grain Attack
This type of pitting aligns with the vertical orientation of the material’s grain structure. The corrosion progresses along the grain boundaries, leading to potential embrittlement and compromised mechanical properties.
Horizontal Grain Attack
Similar to vertical grain attack, horizontal grain attack occurs along the horizontal grain boundaries. This orientation can lead to delamination or layering effects, weakening the material’s cohesion and structural performance.
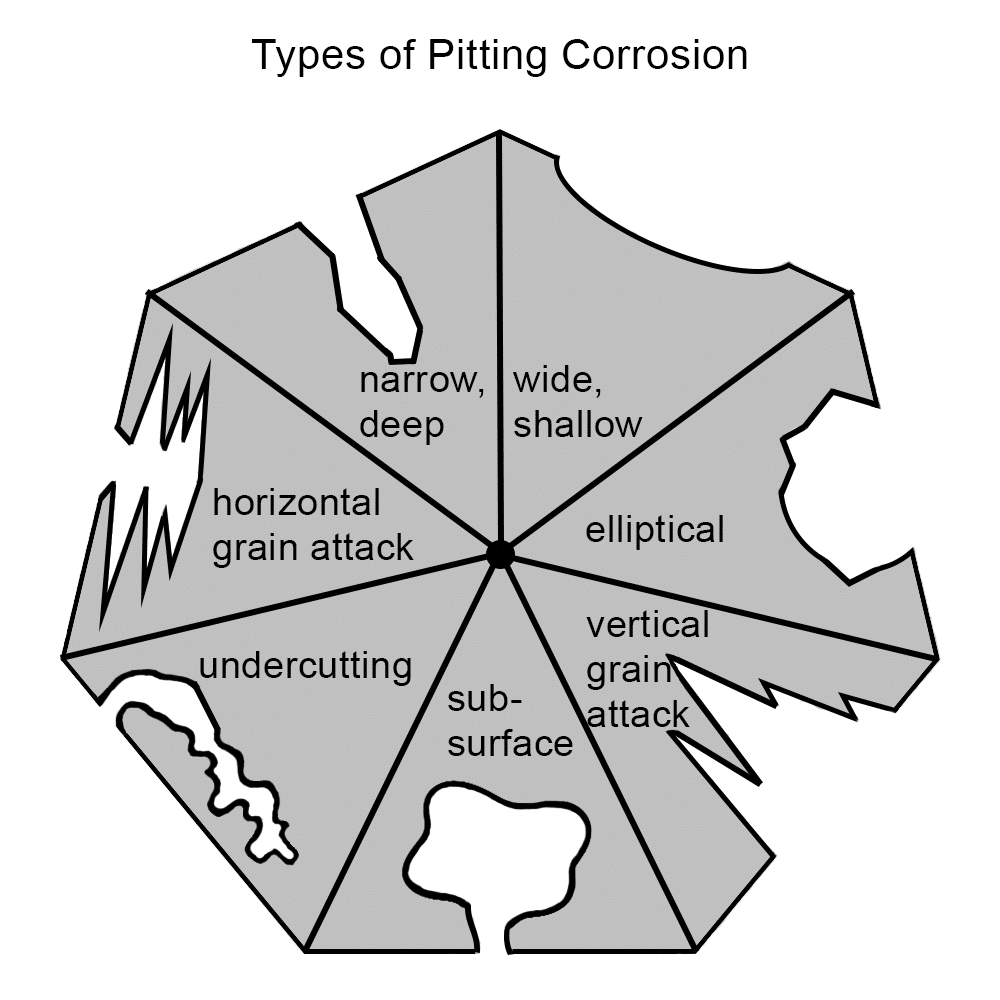
Recognizing these distinct forms of pitting corrosion is valuable for implementing targeted inspection and maintenance strategies, thereby enhancing the longevity of metal surfaces.
The Influence of Surface Area and Microstructure on Pitting Corrosion
Surface area and microstructural composition play a critical role in the initiation and progression of pitting corrosion. Several factors influence susceptibility:
- Small Surface Area Components: In smaller metal components, even a few pits can significantly compromise mechanical integrity, leading to failure under load-bearing conditions.
- Large Surface Area Components: While larger metal surfaces may experience a greater number of pits, the impact on mechanical strength may be less pronounced if pits remain shallow and widely dispersed.
- Surface Finish and Roughness: Scratches, machining marks, and manufacturing defects act as initiation sites for pitting. Polished surfaces with minimal imperfections exhibit greater resistance to localized attack.
- Metallurgical Structure: Variations in grain size, phase distribution, and inclusions can affect pitting resistance. Certain heat treatments can enhance or reduce corrosion resistance depending on alloy composition.
Metals with extensive surface exposure in corrosive environments, such as heat exchangers or marine components, often require additional protective measures to mitigate pitting risks.

After prolonged exposure to saltwater, marine structures like this bridge post bracket often experience pitting corrosion. However, the high density and large surface area of bridge components help maintain structural integrity, reducing the risk of metal failure.
Expanded Causes of Pitting Corrosion
Beyond the commonly known environmental and chemical contributors, several additional factors intensify pitting corrosion:
1. Contaminated Process Fluids
Industrial fluids containing residual chlorides, sulfates, or other aggressive ions can facilitate pitting in metal processing and cooling systems.
2. High-Temperature Exposure
Elevated temperatures increase electrochemical reactivity, accelerating pitting in heat exchangers, boilers, and engine components.
3. Galvanic Interactions
When metals of differing electrochemical potentials are in contact, pitting corrosion may localize on the more anodic material, leading to accelerated degradation.
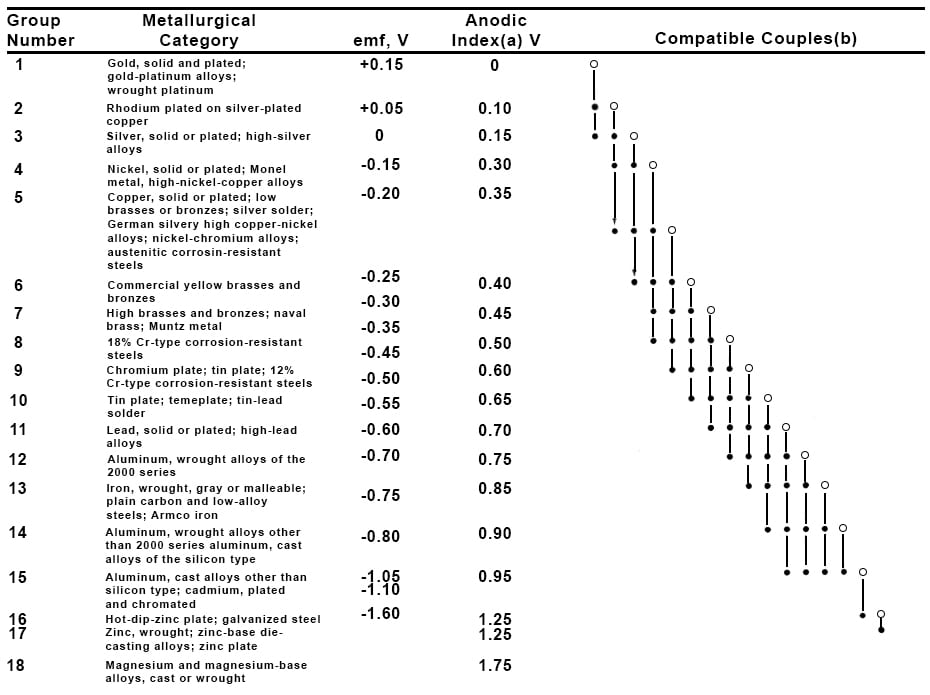
The galvanic series for selected alloys in seawater can help determine which metals of differing electrochemical potentials to avoid using together.
4. Microbial-Induced Corrosion (MIC)
Sulfate-reducing bacteria and other microbial activity can generate acidic byproducts, reducing local pH levels and promoting pitting corrosion in industrial water systems and pipelines.
5. Water Stagnation
Stagnant water allows localized chemical imbalances to develop, concentrating corrosive species and increasing the likelihood of pitting attack.
6. Depletion of Protective Inhibitors
In environments where corrosion inhibitors are utilized, inadequate replenishment of these inhibitors can lead to the breakdown of passive films, exposing the underlying metal to aggressive agents.
Impact of Pitting Corrosion on Engineering Materials
The effect of pitting corrosion varies depending on material composition, environmental exposure, and operational conditions:
- Stainless Steel: Susceptible to pitting in chloride-rich environments, particularly if the passive chromium oxide layer is compromised.
- Aluminum Alloys: Frequently affected in marine and aerospace applications due to their susceptibility to chloride-induced pitting.
- Copper Alloys: While corrosion-resistant under normal conditions, they may experience pitting in stagnant water or aggressive chemical solutions.
- Carbon and Low-Alloy Steels: Prone to pitting when exposed to oxygen-depleted conditions or contaminants in industrial settings.
- Nickel-Based Alloys: Exhibit enhanced pitting resistance in aggressive environments, particularly when alloyed with molybdenum.
Material selection and proper alloying elements, such as molybdenum and nitrogen, enhance resistance to pitting corrosion in high-risk applications.
Detection and Testing Methods for Pitting Corrosion
1. Visual Inspection

Regular inspection of metal surfaces can reveal early signs of pitting corrosion, although subsurface pits may go undetected.
2. Ultrasonic Testing (UT)
Ultrasonic inspection can detect subsurface pitting corrosion by identifying variations in thickness. UT is also commonly used to identify imperfections or defects in metal components.
3. Eddy Current Testing (ECT)

Used primarily in non-ferrous materials, eddy current testing can identify pitting defects without requiring disassembly.
4. Electrochemical Testing
Methods such as potentiodynamic polarization can evaluate a material’s resistance to pitting corrosion under laboratory conditions. Electrochemical Impedance Spectroscopy (EIS) is another common testing method. EIS is a technique used to study how a material resists electrical flow and stores energy when exposed to an alternating current (AC). It helps analyze not only corrosion, but also coatings and battery performance by measuring how the material reacts to different frequencies of electricity.
5. Scanning Electron Microscopy (SEM)
SEM analysis provides high-resolution imaging of pitting morphology, allowing for in-depth assessment of corrosion mechanisms.
Strategies for Preventing Pitting Corrosion
1. Optimized Material Selection
Choosing alloys with good pitting resistance, such as duplex stainless steels and nickel-based alloys, significantly reduces susceptibility in aggressive environments.
2. Environmental Control
Reducing exposure to high chloride concentrations, maintaining controlled humidity, and using deoxygenation techniques in industrial water systems can mitigate corrosion risks.
3. Protective Coatings and Treatments
Applying specialized coatings, such as chromium or nickel, enhances resistance to pitting corrosion by providing a robust protective barrier.
4. Enhanced Surface Finishing
Electropolishing and passivation treatments improve the resistance of stainless steels by enhancing the integrity of the protective oxide layer.
5. Routine Maintenance and Monitoring
Implementing scheduled inspections and utilizing non-destructive testing (NDT) techniques, such as ultrasonic or radiographic testing, can detect early-stage pitting.
6. Use of Corrosion Inhibitors
Chemical inhibitors reduce the aggressiveness of process fluids by forming a passive layer that shields metal surfaces from localized attack.
7. Electrochemical Protection Methods
Cathodic protection systems, including sacrificial anodes and impressed current techniques, effectively prevent pitting in pipelines and marine structures.
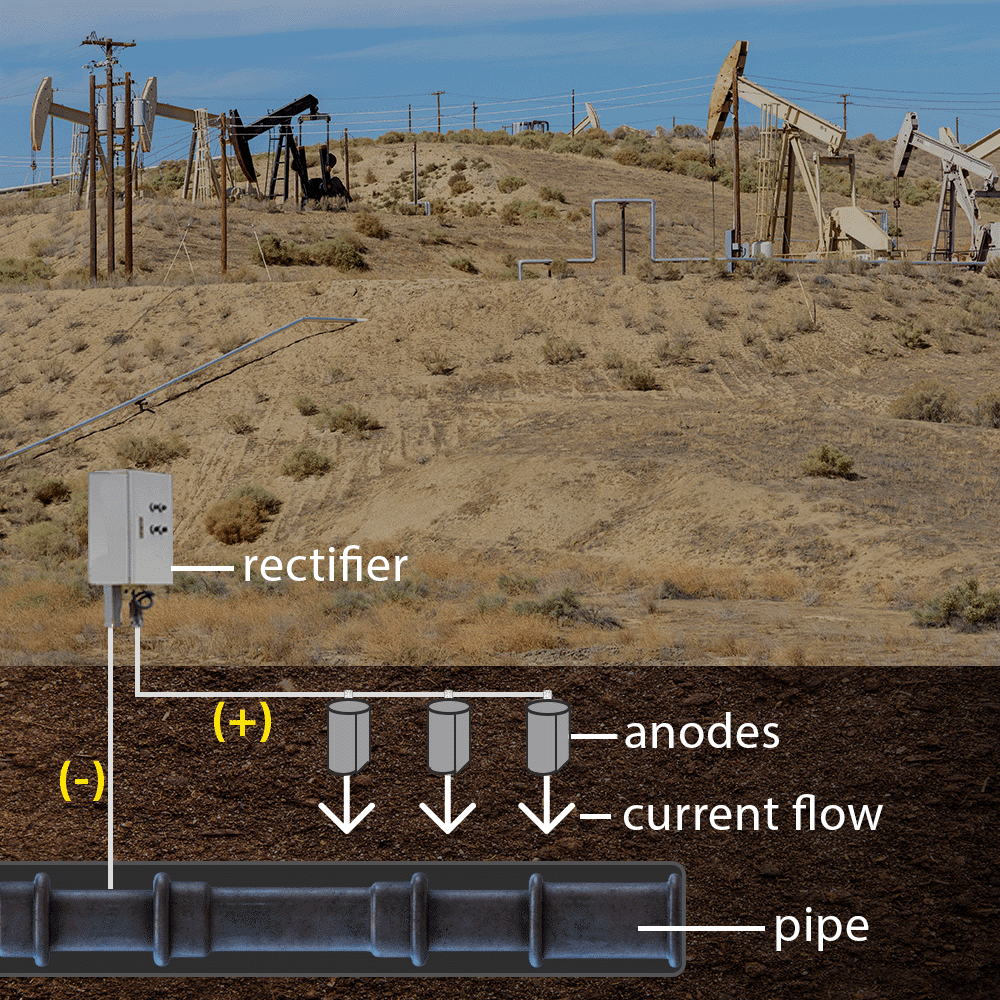
This impressed current cathodic protection (ICCP) system uses an external power source (rectifier) to supply a controlled current to a metal pipe. The inert anodes distribute the protective current, allowing the pipe to remain cathodic.
Pitting corrosion remains one of the more severe and unpredictable forms of localized metal deterioration. Its ability to rapidly compromise mechanical integrity necessitates rigorous preventive strategies across industries. By employing optimized material selection, protective coatings, environmental controls, and proactive maintenance protocols, engineers can significantly extend the lifespan of critical components.
Among the most effective protective solutions, Armoloy Thin Dense Chrome (TDC) provides improved resistance to pitting corrosion, ensuring enhanced durability in extreme conditions. Implementing such protective measures is essential for mitigating financial losses and ensuring safety in corrosion-prone applications.