
From aerospace to food processing, diverse industries utilize metal tools and machinery because of the material’s durability, strength, resistance to heat and repetitive force, as well as cost. However, even the strongest metals used in industrial applications, such as stainless steel, experience degradation over time, which can ultimately lead to metal failure.
Corrosion, abrasion, erosion, and rolling contact fatigue are the most common causes of metal failure. Understanding these four forces—and how to counteract them—is essential to keeping tools, machines, and systems up and running, as designed. Fighting metal failure upfront, by properly designing and manufacturing metal components, will reduce costly maintenance down the road.
In this discussion, we’ll focus on corrosive metal failure—specifically, an overview of the different types of corrosion, how corrosion impacts various industries, and ways to combat corrosive metal failure head-on. Metal failures covered in this article include:
- Stress Corrosion (3 main types)
- Galvanic Corrosion
- Pitting Corrosion
- Biological Corrosion
- Filiform Corrosion
Understanding corrosion
Corrosion is a natural process where metal degrades over time after being exposed to certain chemicals or a specific environment. Rust, one of the most well-known results of corrosion, occurs when iron oxides form on metal. Anyone who has owned a car in an area where they use various types of salt on the roads in winter knows how quickly metal can rust.
Corrosive metal failure can have costly implications, including:
- Decreased effectiveness and accuracy of devices, tools, and machines
- Decreased longevity and increased replacement costs
- Increased downtime
In the fight against metal failure, it helps to understand the various types of corrosion, how they impact various industries, and ways to promote corrosion resistance.
Stress corrosion
Stress corrosion cracking (SCC) is a common metal failure that occurs when the material, often aluminum or stainless steel, is exposed to both tensile stress and a corrosive atmosphere.
While the problem itself can be quite complex, the result of stress corrosion is the growth of a crack formation that penetrates the metal. While most of the material remains untouched, these small cracks can be difficult to detect, and the damage can be hard to ascertain.
There are three main types of stress corrosion:
Chloride stress corrosion
This is a type of intergranular corrosion that occurs when austenitic stainless steel is exposed to a corrosive environment that contains chlorides, such as saltwater, oxygen, and high temperature.
Chloride stress corrosion is a major problem in the aerospace industry because many aircraft are exposed to harsh environments that contain high levels of chloride ions. Derived from saltwater spray, deicing fluids, and coastal fog, chloride ions can penetrate the surface of metal components–including bearings, cylinders, pistons, and valves—leading to damage over time.
Hydrogen stress corrosion
Caused by tensile stress, a susceptible metal, and a hydrogen source, hydrogen stress corrosion creates brittleness and cracking.
For example, due to the harsh operating conditions, duplex stainless steels are widely used in subsea oil and gas extraction equipment. While the duplex stainless steel is strong, metal failures attributed to hydrogen stress corrosion have occurred. Internally, the production fluid can be hot and under high pressure, while the external environment of salt seawater is corrosive, making this industry a prime candidate for hydrogen stress corrosion.
Intergranular stress corrosion
Also known as weld decay, intergranular stress corrosion occurs when some metals reach temperatures between 425°C and 870°C (887°F to 1598°F.). This high-heat environment is common during welding, for example. Under these conditions, the metal grains become separated, leading to decreased strength over time.
Unlike other forms of stress corrosion, this type is microscopic, damaging the material’s structure without necessarily showing surface degradation. Two standards—ASTM A262-15 and ASTM A763-15—help determine the susceptibility of certain metals to intergranular stress corrosion.
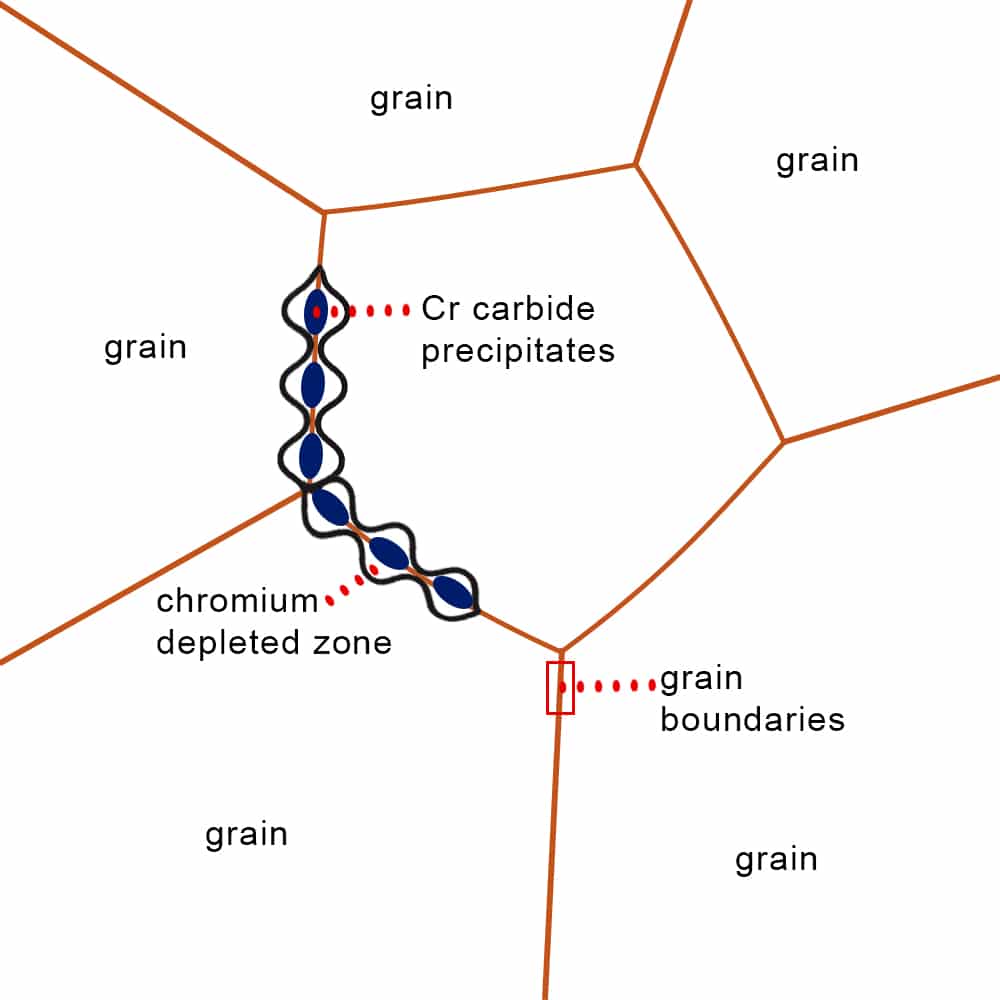
Illustration of intergranular corrosion in grain boundaries of a material
Galvanic corrosion
Galvanic corrosion, also known as dissimilar metal corrosion or bimetallic corrosion, occurs when two different metals are in physical or electrical contact with one another while immersed in an electrolyte (this includes most acids, bases, and salts). In this scenario, the more active metal corrodes faster than the stable metal.
Since a variety of industries and applications, from injection molding to robotics and automation, utilize different metals within the same structure, galvanic corrosion is a widespread concern. Like stress corrosion, galvanic corrosion can cause structural damage and weaken equipment, making it more prone to failure.
However, it is possible to predict and test the effects of this type of corrosion. A galvanic series, outlined in ASTM G82-98, lists metals in order of their corrosion potentials, starting with the most active (electronegative) to the most inactive (electropositive). This series would demonstrate, for example, that if aluminum is in contact with stainless steel, the aluminum may undergo galvanic corrosion because it is the more active metal of the two. Usually, the further apart two metals are in the series, the higher the probability of galvanic corrosion.

galvanic corrosion of a metal fastener
Pitting corrosion
A localized form of corrosion, pitting corrosion causes small, random holes (pits), initiated by de-passivation of the metal’s protective oxide layer. Stainless steel and aluminum, for example, are more susceptible to pitting corrosion because these metals form oxide films on their surfaces naturally.
Direct machining, or an incomplete finishing process that damages surface finishes, can result in pitting. Machined components—such as shafts, bearings, cams, gears, rolls, seals—are susceptible to this type of corrosion; it can be particularly dangerous in load-bearing parts. Pitting damage can also be aesthetic in nature, such as corrosion of stainless steel medical instruments, where a clean, pristine, and new appearance is paramount.
Because pitting corrosion often occurs beneath the metal’s surface, causing minuscule holes that aren’t visible to the naked eye, pitting corrosion is difficult to detect. Once these pits become noticeable, the damage likely has occurred. Although pitting corrosion is repairable, preventing it initially proves more cost-effective. Learn how to prevent pitting corrosion and crevice corrosion in our metal failure mitigation blog.

A severe state of pitting corrosion is seen on this metal plate
Biological corrosion
Also known as biocorrosion, biofouling, and microbial corrosion, biological corrosion occurs when metals are exposed to bacteria or other microorganisms that generate acids, enzymes, and other corrosive substances.
This damage can happen nearly everywhere—in water, on the earth, and in the air. Biological corrosion is present in the carbon and stainless steel pipes and tanks prominent in the food and beverage processing and nuclear power industries, and in the pumps and compressors of water treatment centers.
Like pitting corrosion, biological corrosion is often not apparent until the damage has already occurred. Chromium coatings from Electrolizing excel in these harsh conditions, reducing biological corrosion and increasing product lifespan. Dive deeper into biological corrosion with our in-depth analysis.

Biocorrosion of steel seen at x500 caused by sulfate-reducing bacteria
Filiform corrosion
Thin, worm-like tendrils on a metal’s surface are the hallmarks of filiform corrosion. Filiform corrosion often affects steel, iron, and zinc surfaces covered with paint or other protective coatings and exposed to high-humidity environments. It occurs when moisture penetrates the metal’s coating, causing this type of crevice corrosion.
Aircraft parts, including fuselages and wing components, often experience filiform corrosion because moisture and other corrosive substances, such as salts and acids, expose their surfaces to deterioration.
Also difficult to detect until it is in an advanced stage, filiform corrosion often starts out as a coating defect, like scratches, and or as a weak point, such as beards and holes. Using corrosion-resistant materials and chromium coatings, and keeping the metal dry, can control it without damaging the underlying metal.

Filiform corrosion on a silver aluminum alloy wheel.
Fight metal failure.
The key to corrosive metal failure protection is to stop metal failure before it starts. Armoloy coatings, engineered for improved performance and protection, have undergone corrosion testing using ASTM-B-117 salt spray procedures and have earned acceptance for use in USDA-regulated environments.
To learn more about combating corrosion, explore Analyzing Metal Failure: Corrosion Damage.